732702
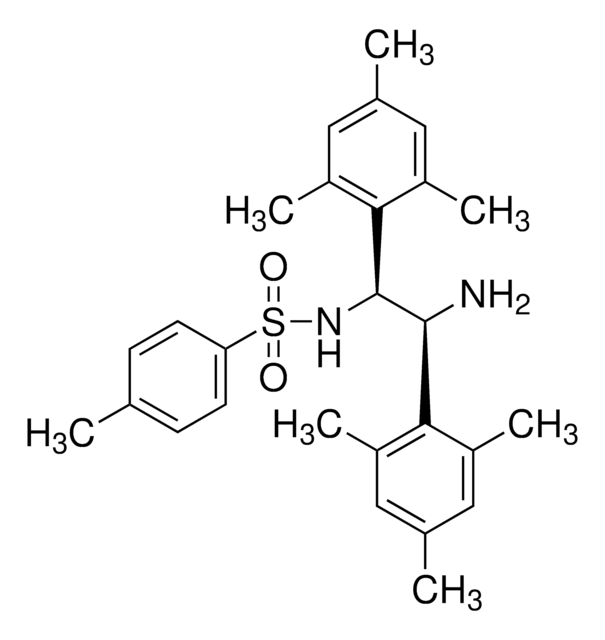
(1S,2S)-N-p-Tosyl-1,2-di(9-anthryl)ethylenediamine
95%
About This Item
Recommended Products
assay
95%
form
solid
mp
218-223 °C
SMILES string
Cc1ccc(cc1)S(=O)(=O)N[C@H]([C@@H](N)c2c3ccccc3cc4ccccc24)c5c6ccccc6cc7ccccc57
InChI
1S/C37H30N2O2S/c1-24-18-20-29(21-19-24)42(40,41)39-37(35-32-16-8-4-12-27(32)23-28-13-5-9-17-33(28)35)36(38)34-30-14-6-2-10-25(30)22-26-11-3-7-15-31(26)34/h2-23,36-37,39H,38H2,1H3/t36-,37-/m0/s1
InChI key
NUBJNKYOQBGKPS-BCRBLDSWSA-N
signalword
Danger
hcodes
pcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Chin group is interested in computational and experimental approaches to understanding stereoselective recognition and catalysis. Their studies in weak forces (H-bonding, electronic and steric effects) has led to a highly efficient method for making limitless varieties of chiral vicinal diamines from the 'mother diamine' that are useful for developing stereoselective organocatalysts or transition metal-based catalysts as well as for developing drugs (Acc Chem Res (2012) p1345). The 'mother diamine' is also useful for making binol, monophos and binap analogs. The Chin group is also interested in using reversible covalent bonds for stereoselective recognition and L to D conversion of natural and non-natural amino acids (EJOC (2012) p229).
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service