710881
Potassium phenoxymethyltrifluoroborate
contains 20% KBr, 97%
About This Item
Recommended Products
assay
97%
form
solid
contains
20% KBr
mp
>300 °C
SMILES string
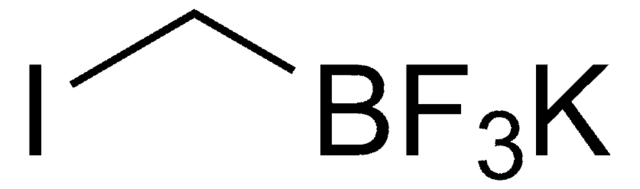
[K+].F[B-](F)(F)COc1ccccc1
InChI
1S/C7H7BF3O.K/c9-8(10,11)6-12-7-4-2-1-3-5-7;/h1-5H,6H2;/q-1;+1
InChI key
OVYSNXCRBZPJIG-UHFFFAOYSA-N
General description
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)