They are normally recovered intact, however, they may disintegrate somewhat in aqueous reactions. Any disintegrated tablets can be removed from the reaction via filtration.
685178
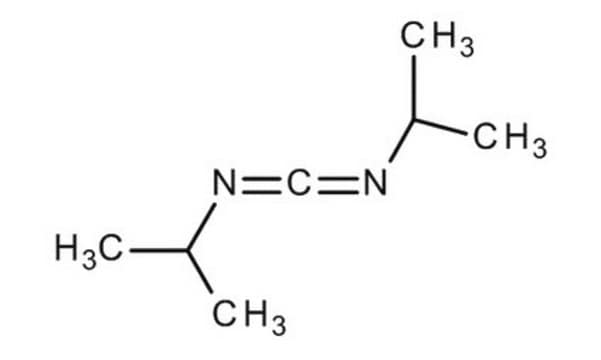
N,N′-Dicyclohexylcarbodiimide, ChemDose™ tablets
Loading: 0.15mmol per tablet
Synonym(s):
ChemDose™, N,N′-Dicyclohexylcarbodiimide impregnated tablets, DCC impregnated tablets
About This Item
Recommended Products
form
tablet
matrix
Magnesium aluminometasilicate base material
InChI
1S/C20H18N2O2/c1-3-22(4-2)13-9-10-16-18(11-13)24-19-12-17(23)14-7-5-6-8-15(14)20(19)21-16/h5-12H,3-4H2,1-2H3
InChI key
VOFUROIFQGPCGE-UHFFFAOYSA-N
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Skin Sens. 1
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
-
Do the ChemDose® tablets disintegrate during the course of the reaction?
1 answer-
Helpful?
-
-
Can I use ChemDose® tablets in any solvent?
1 answer-
Yes, including aqueous solvents.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
Can I use ChemDose® tablets in a microwave or pressure vessel?
1 answer-
Absolutely. Please see the Technical Information for Download section (below) for additional examples of cross-coupling reactions involving the use of microwaves.
Helpful?
-
-
How should I store ChemDose® tablets?
1 answer-
As with most catalysts and ligands, as a general precaution it is best to store them in a refrigerator and/or under an inert atmosphere to maintain activity. Some catalysts, such as Pd(dppf)Cl2·CH2Cl2, are more robust and can be handled in air without issue. Please refer to the ChemDose® label for specific handling of a given catalyst, ligand, or reagent.
Helpful?
-
-
What is the pH stability of the ChemDose® tablets?
1 answer-
The ChemDose® tablets are mildly basic and will dissolve in the presence of mineral acids.
Helpful?
-
-
How should I dispose of spent ChemDose® tablets?
1 answer-
They should be treated as solid waste, contaminated with reaction species. The matrix is non-toxic and environmentally benign.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service