677213
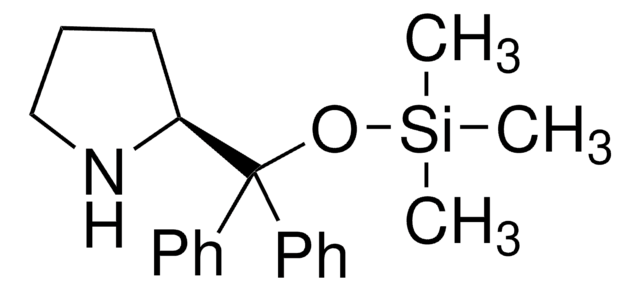
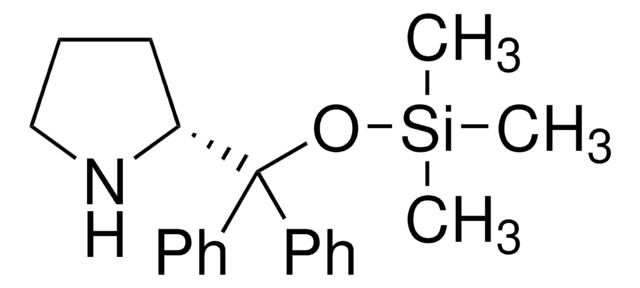
(R)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether
technical grade
Synonym(s):
(R)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]prolinol trimethylsilyl ether, (R)-2-[(Bis(3,5-bis(trifluoromethyl)phenyl)]trimethylsilanyloxy)methyl]pyrrolidine, (R)-2-[(Bis(3,5-bis(trifluoromethyl)phenyl)]trimethylsilyloxy)methyl]pyrrolidine
About This Item
Recommended Products
grade
technical grade
optical purity
enantiomeric excess: ≥99.0% (HPLC)
mp
46-55 °C
SMILES string
C[Si](C)(C)OC([C@H]1CCCN1)(c2cc(cc(c2)C(F)(F)F)C(F)(F)F)c3cc(cc(c3)C(F)(F)F)C(F)(F)F
InChI
1S/C24H23F12NOSi/c1-39(2,3)38-20(19-5-4-6-37-19,13-7-15(21(25,26)27)11-16(8-13)22(28,29)30)14-9-17(23(31,32)33)12-18(10-14)24(34,35)36/h7-12,19,37H,4-6H2,1-3H3/t19-/m1/s1
InChI key
MOHRGTBNEJKFMB-LJQANCHMSA-N
Application
- Cyclocondensation of enals with methylenepyrrolidines
- Organocatalytic additions of β-ketosulfoxides to conjugated aldehydes
- Organocatalytic aza-Michael reactions
- Stereoselective propargylic alkylation of propargylic esters with aldehydes
- Epoxidation or aziridination of α,β-unsaturated aldehydes and Feist-Benary reactions of 1,3-dicarbonyls
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
>230.0 °F - closed cup
flash_point_c
> 110 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Karl Anker Jørgensen and his group have developed ethers which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
Professor Geoffrey Coates and co-workers at Cornell University have reported the preparation and use of catalysts composed of an oxophilic Lewis acid and a cobalt tetracarbonyl anion for the ring expansive carbonylation of epoxides to b-lactones and b-lactones to succinic anhydrides.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)


![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol ≥99.0%](/deepweb/assets/sigmaaldrich/product/structures/201/440/11d18670-8609-4657-bb4b-af6c424f8791/640/11d18670-8609-4657-bb4b-af6c424f8791.png)

![(S)-2-[[3,5-Bis(trifluoromethyl)phenyl]thioureido]-N-benzyl-N,3,3-trimethylbutanamide 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)


