All Photos(1)
About This Item
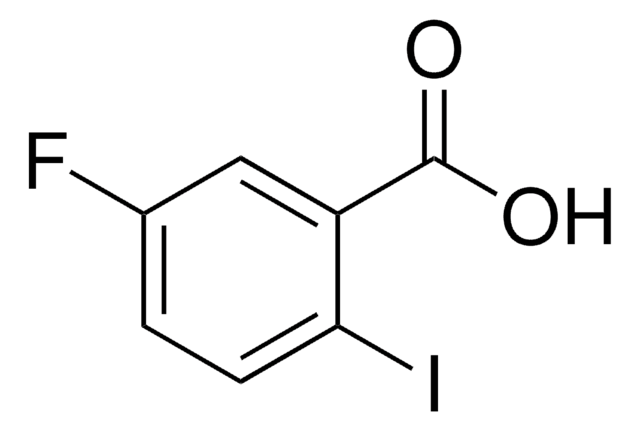
Linear Formula:
C6H3FICOOH
CAS Number:
Molecular Weight:
266.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
164-168 °C (lit.)
SMILES string
OC(=O)c1cc(I)ccc1F
InChI
1S/C7H4FIO2/c8-6-2-1-4(9)3-5(6)7(10)11/h1-3H,(H,10,11)
InChI key
QNNJHBNTHVHALE-UHFFFAOYSA-N
Application
2-Fluoro-5-iodobenzoic acid may be used in the preparation of 1-N-ethyl 6-iodoquinolonic acid and 1-N-cyclopropyl 6-iodoquinolonic acid. It may also be used as a precursor for the generation of electron transfer dissociation (ETD) reagents.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and biological activity of 4 ″-O-acyl derivatives of 14-and 15-membered macrolides linked to ω-quinolone-carboxylic unit.
Skugor MM, et al.
Bioorganic & Medicinal Chemistry, 18(17), 6547-6558 (2010)
Electron-transfer reagent anion formation via electrospray ionization and collision-induced dissociation.
Huang TY, et al.
Analytical Chemistry, 78(21), 7387-7391 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service