All Photos(1)
About This Item
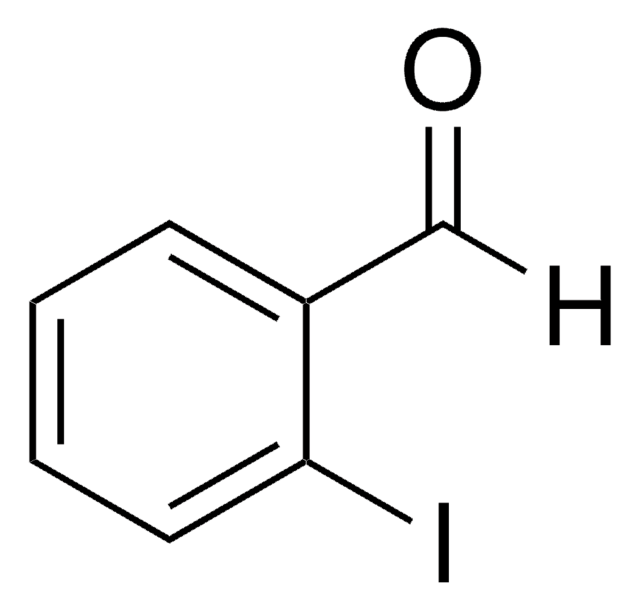
Linear Formula:
IC6H4CHO
CAS Number:
Molecular Weight:
232.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
57-60 °C (lit.)
functional group
aldehyde
iodo
storage temp.
2-8°C
SMILES string
Ic1cccc(C=O)c1
InChI
1S/C7H5IO/c8-7-3-1-2-6(4-7)5-9/h1-5H
InChI key
RZODAQZAFOBFLS-UHFFFAOYSA-N
General description
3-Iodobenzaldehyde can be prepared by the iodination of benzaldehyde using 1,3-diiodo-5,5-dimethylhydantoin in sulfuric acid.
Application
3-Iodobenzaldehyde may be used as a starting material in the preparation of:
- 3-iodocinnamic acid
- 3-(3-hydroxy-3-methylbut-1-yn-1-yl)benzaldehyde
- 1,3-dihydroxy-2-(3-iodophenyl)-4,4,5,5-tetramethylimidazolidine
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and evaluation of potent and selective human V1a receptor antagonists as potential ligands for PET or SPECT imaging.
Fabio K, et al.
Bioorganic & Medicinal Chemistry, 20(3), 1337-1345 (2012)
Cross-coupling of aryl iodides with paramagnetic terminal acetylenes derived from 4, 4, 5, 5-tetramethyl-2-imidazoline-1-oxyl 3-oxide.
Klyatskaya SV, et al.
Russian Chemical Bulletin, 51(1), 128-134 (2002)
Suzuki Reactions with Stable Organic Radicals-Synthesis of Biphenyls Substituted with Nitronyl-Nitroxide Radicals.
Stroh C, et al.
European Journal of Organic Chemistry, 2005(17), 3697-3703 (2005)
1, 3-Diiodo-5,5-dimethylhydantoin-An efficient reagent for iodination of aromatic compounds.
Chaikovskii VK, et al.
Russ. J. Org. Chem., 43(9), 1291-1296 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service