All Photos(1)
About This Item
Linear Formula:
HOCH2CH2CH[NHCO2C(CH3)3]CH2OH
CAS Number:
Molecular Weight:
205.25
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
optical activity
[α]20/D −8°, c = 1 in chloroform
mp
65-69 °C (lit.)
SMILES string
CC(C)(C)OC(=O)N[C@H](CO)CCO
InChI
1S/C9H19NO4/c1-9(2,3)14-8(13)10-7(6-12)4-5-11/h7,11-12H,4-6H2,1-3H3,(H,10,13)/t7-/m0/s1
InChI key
KLRRFBSWOIUAHZ-ZETCQYMHSA-N
Application
(S)-(−)-2-(Boc-amino)-1,4-butanediol can be used as a reactant to synthesize:
- Thiourea-based organocatalysts for asymmetric Michael addition reactions of nitroalkenes to α-nitrocyclohexanone.
- Bis-copper (II) complex based catalysts for enantioselective Michael reactions.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Asymmetric Michael additions of α-nitrocyclohexanone to aryl nitroalkenes catalyzed by natural amino acid-derived bifunctional thioureas
Jo?rres M, et al.
Organic Letters, 14(17), 4518-4521 (2012)
Copper (II) in organic synthesis. XI. Evaluation of the ligand architecture on the efficiency of a copper (II) catalyst for enantioselective Michael reactions
Desimoni G, et al.
Tetrahedron, 51(14), 4131-4144 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service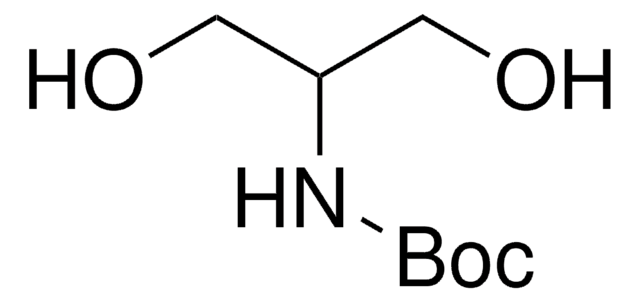



![Trimethylolpropane tris[poly(propylene glycol), amine terminated] ether average Mn 440](/deepweb/assets/sigmaaldrich/product/structures/186/658/1b1d510a-705a-4bfd-b90a-9dec80d64467/640/1b1d510a-705a-4bfd-b90a-9dec80d64467.png)