532363
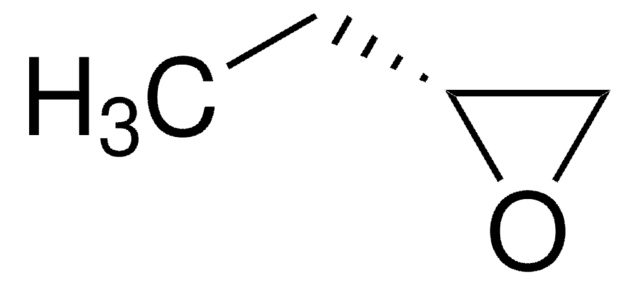
(S)-(−)-1,2-Epoxybutane
98%
Synonym(s):
(2S)-Ethyloxirane
Select a Size
$215.00
Available to ship onMay 05, 2025Details
Notify Me
Get notified when this item is ready to ship via email.
Select a Size
About This Item
$215.00
Available to ship onMay 05, 2025Details
Notify Me
Get notified when this item is ready to ship via email.
Recommended Products
assay
98%
optical activity
[α]20/D −10°, neat
refractive index
n20/D 1.386 (lit.)
bp
63 °C (lit.)
density
0.837 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
CC[C@H]1CO1
InChI
1S/C4H8O/c1-2-4-3-5-4/h4H,2-3H2,1H3/t4-/m0/s1
InChI key
RBACIKXCRWGCBB-BYPYZUCNSA-N
Related Categories
Application
- As a starting material to prepare (+)- and (−)-homononactic acids, which are used as intermediates in the total synthesis of a cyclic antibiotic tetranactin.[1]
- To prepare a chiral phosphorus synthon, which is applicable in the synthesis of phytoprostane B1 type I.[2]
- To prepare Eu3+-based precatalysts applicable in the Mukaiyama Aldol reaction in water.[3]
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
10.0 °F - closed cup
flash_point_c
-12.2 °C - closed cup
ppe
Faceshields, Gloves, Goggles
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service