All Photos(1)
About This Item
Empirical Formula (Hill Notation):
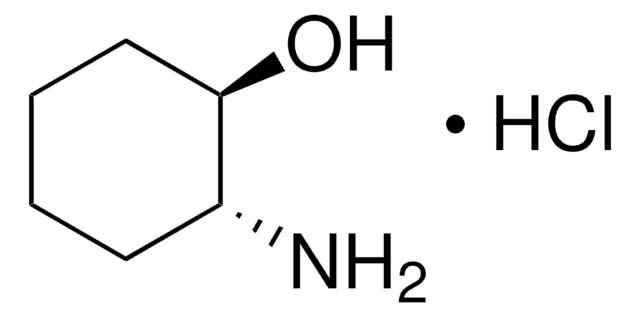
C5H11NO · HCl
CAS Number:
Molecular Weight:
137.61
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
191-196 °C (lit.)
SMILES string
Cl.N[C@H]1CCC[C@@H]1O
InChI
1S/C5H11NO.ClH/c6-4-2-1-3-5(4)7;/h4-5,7H,1-3,6H2;1H/t4-,5-;/m0./s1
InChI key
ZFSXKSSWYSZPGQ-FHAQVOQBSA-N
General description
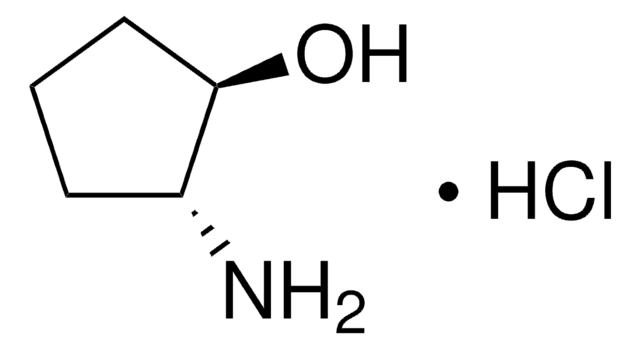
trans-2-Aminocyclopentanol hydrochloride is an aminocyclanol. Its d,l-cis- and d,l-trans- forms have been synthesized. The trans-form of the product can be produced in large (multigram) scale via carbamate addition protocol. Cholinesterase inhibitory potential of cis-form of 2-aminocyclopentanol hydrochloride is higher (twofold) that of its trans-form.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Stereochemistry of Aminocyclanols. Synthesis of cis Epimers via Oxazolines. The 2-Aminocyclopentanols*.
McCasland GE and Smith DA.
Journal of the American Chemical Society, 72(5), 2190-2195 (1950)
James A Birrell et al.
Organic letters, 15(12), 2895-2897 (2013-06-08)
A highly enantioselective addition of phenyl carbamate to meso-epoxides has been developed to efficiently generate protected trans-1,2-amino alcohols. This transformation is promoted by an oligomeric (salen)Co-OTf catalyst and has been used to prepare two useful 2-aminocycloalkanol hydrochlorides in enantiopure form
Preparation of antidotes for anticholinesterase poisoning. IV. Synthesis and protective effectiveness of 2'-(cis-and trans-2'-hydroxycyclohexyl) aminoethyl 1-phenylcyclopentanecarboxylate hydrochlorides.
Bannard RAB and Parkkari JH.
Canadian Journal of Chemistry, 48(9), 1377-1382 (1970)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service