464015
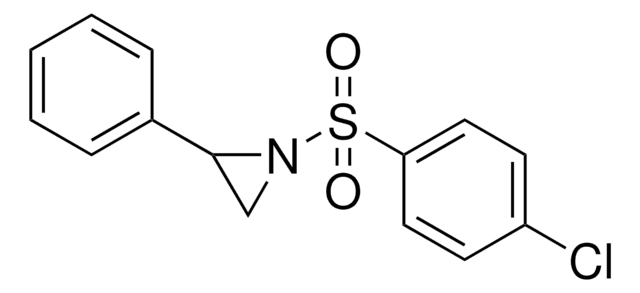
(S)-(+)-2-Benzyl-1-(p-tolylsulfonyl)aziridine
98%
Synonym(s):
(S)-2-Benzyl-1-tosylaziridine
About This Item
Recommended Products
assay
98%
optical activity
[α]20/D +8.8°, c = 1.3 in toluene
mp
92-94 °C (lit.)
functional group
phenyl
sulfonamide
SMILES string
Cc1ccc(cc1)S(=O)(=O)N2C[C@@H]2Cc3ccccc3
InChI
1S/C16H17NO2S/c1-13-7-9-16(10-8-13)20(18,19)17-12-15(17)11-14-5-3-2-4-6-14/h2-10,15H,11-12H2,1H3/t15-,17?/m0/s1
InChI key
ISURUORMAKCTFF-MYJWUSKBSA-N
Application
- To prepare tert-butyl(S)-6-((4-methylphenyl)sulfonamido)-3-oxo-7-phenylheptanoate, an intermediate for the synthesis of substituted octahydroindoles.[1]
- In the preparation of ortho-bromo phenethylamine products, which are further used to synthesize chiral 2-substituted indolines.[2]
- In the synthesis of β-aryltelluro amines as potent carbonic anhydrase inhibitors.[3]
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Tris[2-(methylamino)ethyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/217/368/3e89e134-669e-4a03-9a34-f97b50399bb2/640/3e89e134-669e-4a03-9a34-f97b50399bb2.png)