All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H4ClNO3S
CAS Number:
Molecular Weight:
217.63
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
mp
148-152 °C (lit.)
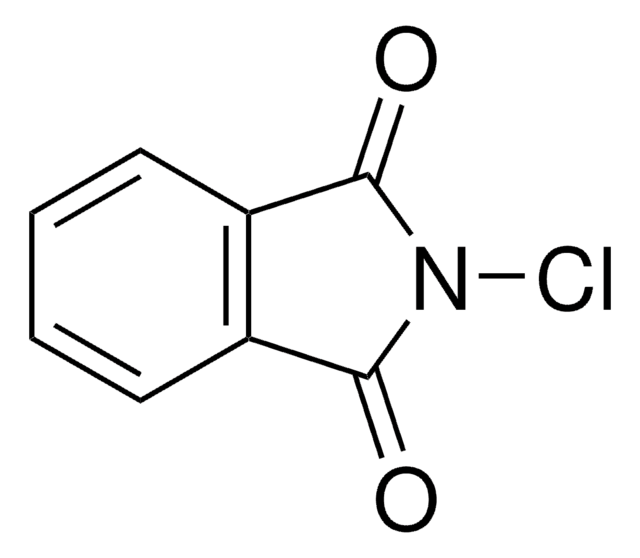
SMILES string
ClN1C(=O)c2ccccc2S1(=O)=O
InChI
1S/C7H4ClNO3S/c8-9-7(10)5-3-1-2-4-6(5)13(9,11)12/h1-4H
InChI key
VKWMGUNWDFIWNW-UHFFFAOYSA-N
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Jayasree et al.
Journal - Association of Official Analytical Chemists, 70(4), 762-763 (1987-07-01)
Three simple titrimetric methods have been developed to determine iodine-bromine numbers of some edible oils, such as coconut, gingelly, groundnut, mustard, olive, palm olein, and sunflower, using 3 N-chloroimides. The 3 N-chloroimides are N-chlorophthalimide, N-chlorosuccinimide, and N-chlorosaccharin, all of which
N-chlorosaccharin as a possible chlorinating reagent: structure, chlorine potential, and stability in water and organic solvents.
H S Dawn et al.
Journal of pharmaceutical sciences, 59(7), 955-959 (1970-07-01)
Kevin I Booker-Milburn et al.
Organic letters, 5(18), 3313-3315 (2003-08-29)
[reaction: see text] N-Chlorosaccharin has been shown to undergo electrophilic Ritter-type reactions with alkenes in acetonitrile. The resulting labile beta-chloro sulfonylamidines can be ring-opened and cyclized to imidazolines. Overall this provides a one pot method for the electrophilic diamination of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service