All Photos(1)
About This Item
Linear Formula:
BrC6H3(I)CO2H
CAS Number:
Molecular Weight:
326.91
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
219-221 °C (lit.)
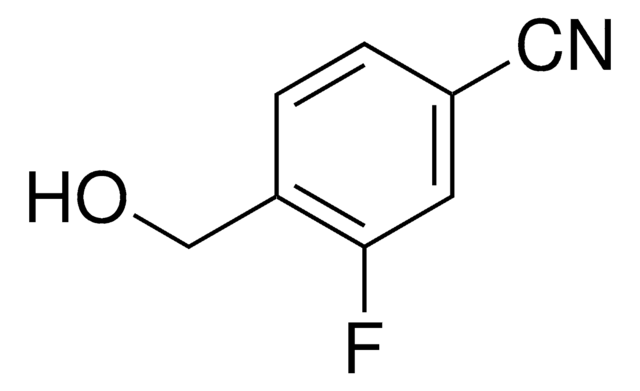
SMILES string
OC(=O)c1cc(Br)cc(I)c1
InChI
1S/C7H4BrIO2/c8-5-1-4(7(10)11)2-6(9)3-5/h1-3H,(H,10,11)
InChI key
MKJBJYCBKXPQSY-UHFFFAOYSA-N
General description
3-Bromo-5-iodobenzoic acid (BrIBA) is a halogen substituted carboxylic acid.
Application
3-Bromo-5-iodobenzoic acid may be used in the preparation of the following:
- Phenyl(3-bromo-5-iodo)benzoate.
- As starting reagent for the large-scale synthesis of the thromboxane receptor antagonist 3-{3-[2-(4-chlorobenzenesulfonamido)ethyl]-5-(4-fluorobenzyl)phenyl}propionic acid, via regioselective Heck cross-coupling reaction.
- Methyl 3-bromo-5-iodobenzoate.
- 3-Bromo-5-(triisopropylsilylethynyl)benzoic acid, via Sonogashira coupling reaction.
- Trifluoroacetophenone.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of acetylene-functionalized [2]rotaxane monomers directed toward side chain-type polyrotaxanes.
Nakazono K, et al.
Polymer Journal, 42(3), 208-215 (2010)
S Lindman et al.
Bioorganic & medicinal chemistry, 8(9), 2375-2383 (2000-10-12)
Rigidification of peptides by cyclization and iterative incorporation of well-defined secondary structure mimetics constitutes one approach to the design of non-peptidergic structures with better defined conformations. We herein present the synthesis of a potential gamma-turn mimetic scaffold, and its incorporation
A scalable synthesis of the thromboxane receptor antagonist 3-{3-[2-(4-chlorobenzenesulfonamido) ethyl]-5-(4-fluorobenzyl) phenyl} propionic acid via a regioselective Heck cross-coupling strategy.
Waite DC and Mason CP.
Organic Process Research & Development, 2(2), 116-120 (1998)
Keisuke Gondo et al.
Molecules (Basel, Switzerland), 17(6), 6625-6632 (2012-06-26)
Reaction of dibenzoylmethane with (diacetoxyiodo)benzene in the presence of KOH in MeCN quantitatively gave the corresponding iodonium ylide, which was treated with a HF reagent to afford the corresponding 2-fluorinated dibenzoylmethane in 14-50% yields. The similar reaction of the iodonium
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![3-[4-(Dimethylamino)phenyl]propanoic acid hydrochloride AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/161/863/4d4f0008-ec42-42c7-9f1f-c03aec8be0e0/640/4d4f0008-ec42-42c7-9f1f-c03aec8be0e0.png)