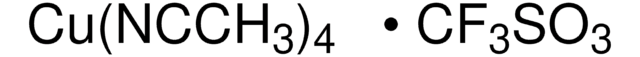All Photos(2)
About This Item
Linear Formula:
(O=)C8H13CO2C2H5
CAS Number:
Molecular Weight:
196.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
liquid
refractive index
n20/D 1.483 (lit.)
bp
85-87 °C/0.1 mmHg (lit.)
density
1.04 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C1CCCCCCC1=O
InChI
1S/C11H18O3/c1-2-14-11(13)9-7-5-3-4-6-8-10(9)12/h9H,2-8H2,1H3
InChI key
OJCDCHCBNASPBS-UHFFFAOYSA-N
General description
Ethyl 2-oxo-1-cyclooctanecarboxylate (2-carbethoxycyclooctanone) is a β-keto ester. It undergoes Michael addition with 3-buten-2-one (methyl vinyl ketone, MVK) in the presence of potassium bis(1,2-benzenediolato)phenylsilicate to afford the corresponding 1,4-adduct. It can be synthesized from cycloheptanone. Its boiling point, density and refractive index have been determined.
Application
Ethyl 2-oxo-1-cyclooctanecarboxylate (2-carbethoxycyclooctanone) may be used in the synthesis of 2-carbethoxy-2-cyclooctenone.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Activated cyclooctenones are effective dienophiles.
Liu HJ, et al.
Canadian Journal of Chemistry, 75(6), 899-912 (1997)
Pentacoordinate Organosilicate-Catalyzed Michael Addition of ?-Keto Esters to 3-Buten-2-one.
Tateiwa J-U and Hosomi A.
European Journal of Organic Chemistry, 2001(8), 1445-1448 (2001)
Yaws CL.
The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 336-336 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service