383449
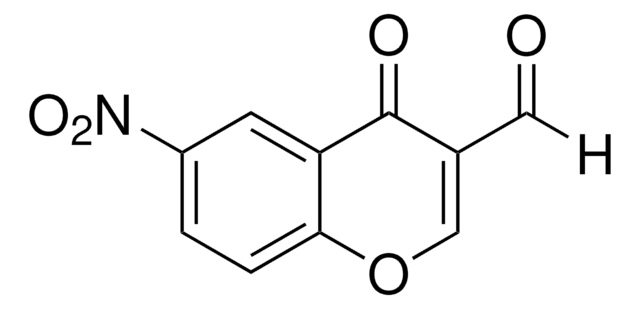
3-Formylchromone
97%
Synonym(s):
4-Oxo-4H-1-benzopyran-3-carboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H6O3
CAS Number:
Molecular Weight:
174.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
151-153 °C (lit.)
SMILES string
O=CC1=COc2ccccc2C1=O
InChI
1S/C10H6O3/c11-5-7-6-13-9-4-2-1-3-8(9)10(7)12/h1-6H
InChI key
FSMYWBQIMDSGQP-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770)
General description
Electrospray ionization mass spectrometry (ESI-MS) of protonated 3-formylchromone (3-FC) shows loss of H2 as a major fragmentation route to yield a ketene cation, which on reaction with water forms a protonated carboxylic acid. The invivo salubrious effects of 3-FC against nitrosodiethylamine (NDEA) mediated early hepatocellular carcinogenesis has been investigated. Synthesis and characterization of 3-FC and its derivatives has been reported.
Application
3-Formylchromone may be used in the following studies:
- Preparation of library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones, by three-component domino reactions with (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones and anilines under catalyst-free conditions.
- Facile and ecofriendly synthesis of new chromonyl chalcones.
- Synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andrey S Plaskon et al.
The Journal of organic chemistry, 73(15), 6010-6013 (2008-07-03)
A facile and versatile procedure for the synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones was elaborated on the basis of TMSCl-mediated recyclization of 3-formylchromone with various anilines. Limitations and scope of this methodology were established, and a possible mechanism for the heterocyclizations
Pitchaimani Prasanna et al.
Beilstein journal of organic chemistry, 10, 459-465 (2014-03-13)
The three-component domino reactions of (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones, 3-formylchromone and anilines under catalyst-free conditions afforded a library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones in good to excellent yields and in a diastereoselective transformation. This transformation generates one C-C and one C-N bond and presumably proceeds
Zeba N Siddiqui et al.
Journal of enzyme inhibition and medicinal chemistry, 27(1), 84-91 (2011-05-27)
A facile and ecofriendly synthesis of new chromonyl chalcones 3a-b from 3-formylchromone 1 and active methyl compounds 2a-b is reported under thermal solvent-free heating condition in good yields. The chromonyl chalcones 3a-b were used as intermediates under green condition for
Muhammed Bilaal Ismail et al.
Nucleosides, nucleotides & nucleic acids, 38(12), 950-971 (2019-07-11)
Herein, we report the DNA interaction studies of rhenium(I) and -(V) compounds with Schiff base chelates encompassing biologically relevant moieties. More specifically, the DNA interaction capabilities of these rhenium complexes were probed using Gel Electrophoresis and Calf Thymus-DNA titrations monitored
Koichi Takao et al.
Bioorganic chemistry, 83, 432-437 (2018-11-15)
A series of eighteen pyrano[4,3-b][1]benzopyranone derivatives (1a-9b) were synthesized, and structure-activity relationships of their monoamine oxidase (MAO) A and B, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) inhibitory activities were evaluated. Most of the synthesized compounds exhibited weak inhibitory activity toward MAO-A
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service