All Photos(4)
About This Item
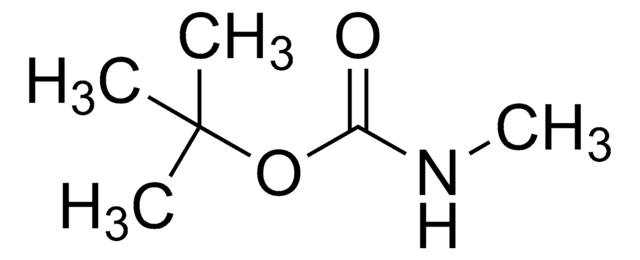
Linear Formula:
CH3C6H4SO2NHCO2C(CH3)3
CAS Number:
Molecular Weight:
271.33
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
mp
121-123 °C (lit.)
solubility
chloroform: soluble 25 mg/mL, clear, colorless
SMILES string
Cc1ccc(cc1)S(=O)(=O)NC(=O)OC(C)(C)C
InChI
1S/C12H17NO4S/c1-9-5-7-10(8-6-9)18(15,16)13-11(14)17-12(2,3)4/h5-8H,1-4H3,(H,13,14)
InChI key
DUTLOVSBVBGNDM-UHFFFAOYSA-N
General description
N-(tert-Butoxycarbonyl)-p-toluenesulfonamide is a N-substituted sulphonamide and its reaction with N-trityl L-serine esters under Mitsunobu reaction conditions is reported. It can be directly coupled with primary, secondary and allylic alcohols under Mitsunobu conditions to afford various sulfonyl-protected amines.
Application
N-(tert-Butoxycarbonyl)-p-toluenesulfonamide may be used in the preparation of enyne amide, precursor for Pauson-Khand reaction.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tetrahedron Letters, 30, 5709-5709 (1989)
Toshio Honda et al.
The Journal of organic chemistry, 72(17), 6541-6547 (2007-07-31)
Diastereoselective formal synthesis of a monoterpene alkaloid, (-)-incarvilline, the key intermediate for the synthesis of (-)-incarvillateine, was achieved by using an intramolecular Pauson-Khand reaction of (S)-N-[(E)-2-butenyl]-N-(3-butynyl-2-methoxymethoxy)-p-toluenesulfonamide as a key step.
Use of the Mitsunobu reaction in the synthesis of orthogonally protected a, ?-diaminopropionic acids.
Kelleher F.
Tetrahedron Letters, 48(28), 4879-4882 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service