192805
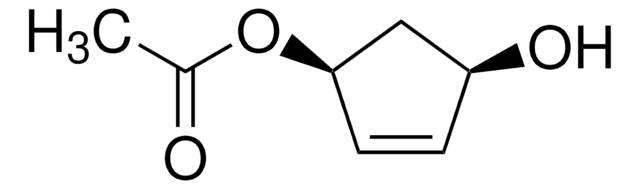
1,3-Cyclopentanediol, mixture of cis and trans
95%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C5H8(OH)2
CAS Number:
Molecular Weight:
102.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
95%
form
liquid
refractive index
n20/D 1.484 (lit.)
bp
80-85 °C/0.1 mmHg (lit.)
mp
40 °C (lit.)
density
1.094 g/mL at 25 °C (lit.)
SMILES string
OC1CCC(O)C1
InChI
1S/C5H10O2/c6-4-1-2-5(7)3-4/h4-7H,1-3H2
InChI key
NUUPJBRGQCEZSI-UHFFFAOYSA-N
Related Categories
Application
1,3-Cyclopentanediol was used in the synthesis of 1,3-cyclopentanediol bis(4-methylbenzenesulfonate) and β-hydroxy-substituted cyclic carbonyl compounds. It was also used in direct enantiomer resolution of diols without derivatization on a chiral polysiloxane by capillary gas chromatography.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stepwise Introduction of p-Electron Cross-Conjugation: A Possible Access to [5] Radialenes?
Geneste F, et al.
The Journal of Organic Chemistry, 62(16), 5339-5343 (1997)
Direct asymmetric a-hydroxylation of 2-hydroxymethyl ketones.
Paju A, et al.
Tetrahedron, 58(36), 7321-7326 (2002)
Direct resolution of enantiomeric diols by capillary gas chromatography on a chiral polysiloxane derived from (R, R)-tartramide.
Nakamura K, et al.
Analytical Chemistry, 62(5), 539-541 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service