All Photos(1)
About This Item
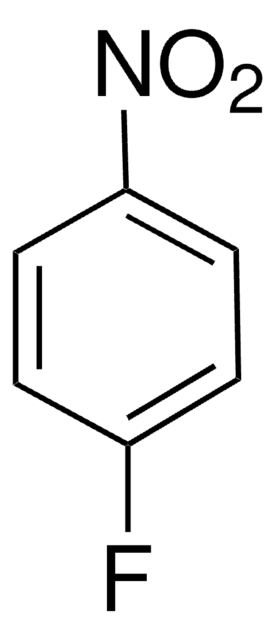
Linear Formula:
FC6H4OCH3
CAS Number:
Molecular Weight:
126.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
liquid
refractive index
n20/D 1.488 (lit.)
bp
158 °C/743 mmHg (lit.)
density
1.104 g/mL at 25 °C (lit.)
SMILES string
COc1cccc(F)c1
InChI
1S/C7H7FO/c1-9-7-4-2-3-6(8)5-7/h2-5H,1H3
InChI key
MFJNOXOAIFNSBX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Fluoroanisole was used in the synthesis of 4-fluoro-5,6-dihydroxytryptamine. It was also used in the synthesis of 3-fluoro- and 5-fluoronoradrenaline.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and EPR investigations of fluorocatecholamines.
Stegmann HB, et al.
Journal of the Chemical Society. Perkin Transactions 1, 2(3), 547-555 (1994)
M Kawase et al.
Journal of medicinal chemistry, 33(8), 2204-2211 (1990-08-01)
The 5,6-dihydroxytryptamine (5,6-DHT) derivatives 4-fluoro- and 7-fluoro-5,6-DHTs (26a,b) and 4,7-difluoro-5,6-DHT (26c) were synthesized from 3-fluoroanisole (1) and 1,4-difluoro-2,3-dimethoxybenzene (13), respectively. Efficient methods were developed for the conversion of 1 to 4-fluoro- and 7-fluoro-5,6-bis(benzyloxy)indoles (12a,b, respectively), and 13 to 4,7-difluoro-5,6-[( diphenylmethylene)dioxy]indole
Oriol Planas et al.
Science (New York, N.Y.), 367(6475), 313-317 (2020-01-18)
Bismuth catalysis has traditionally relied on the Lewis acidic properties of the element in a fixed oxidation state. In this paper, we report a series of bismuth complexes that can undergo oxidative addition, reductive elimination, and transmetallation in a manner
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service