15406
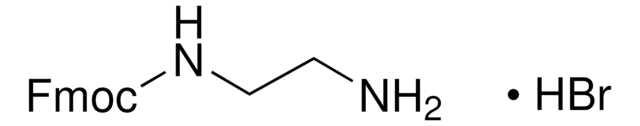
N-Boc-cadaverine
≥97.0% (NT)
Synonym(s):
N-Boc-1,5-diaminopentane, tert-Butyl N-(5-aminopentyl)carbamate
About This Item
Recommended Products
Quality Level
assay
≥97.0% (NT)
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.460
density
0.972 g/mL at 20 °C (lit.)
functional group
Boc
amine
SMILES string
NCCCCCNC(OC(C)(C)C)=O
InChI
1S/C10H22N2O2/c1-10(2,3)14-9(13)12-8-6-4-5-7-11/h4-8,11H2,1-3H3,(H,12,13)
InChI key
DPLOGSUBQDREOU-UHFFFAOYSA-N
Application
- Synthesis of of a supermacrocycle that self-assemble to form organic nanotubes.
- Preparation of water-soluble unsymmetrical sulforhodamine fluorophores from monobrominated sulfoxanthene dye.
- Synthesis of functionalized porphyrins as biocompatible carrier system for photodynamic therapy (PDT).
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
228.2 °F - closed cup
flash_point_c
109.0 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service











![2-[2-(Boc-amino)ethoxy]ethanol 97%](/deepweb/assets/sigmaaldrich/product/structures/413/416/884359e5-1cb4-4071-bb4f-28d9844db662/640/884359e5-1cb4-4071-bb4f-28d9844db662.png)
