All Photos(1)
About This Item
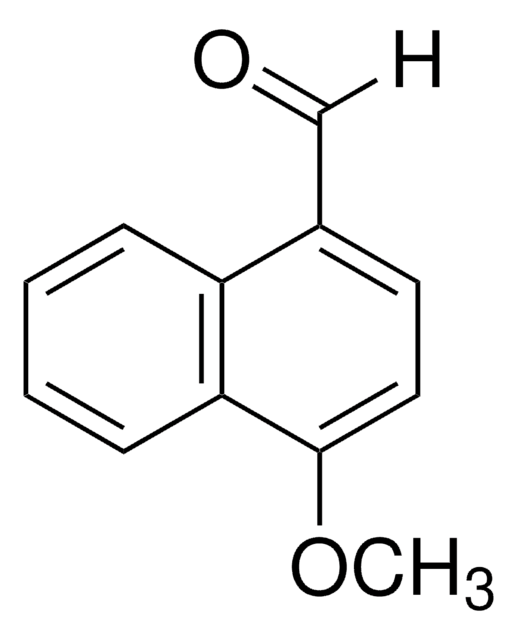
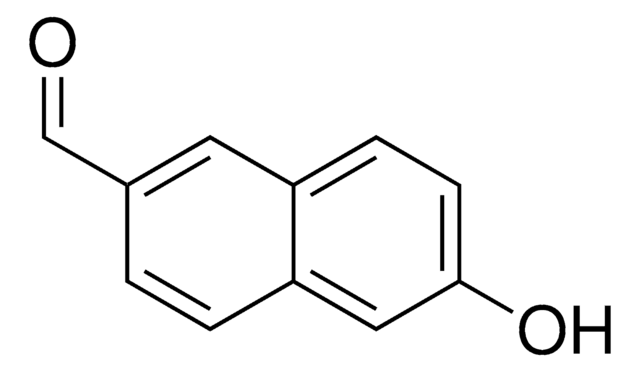
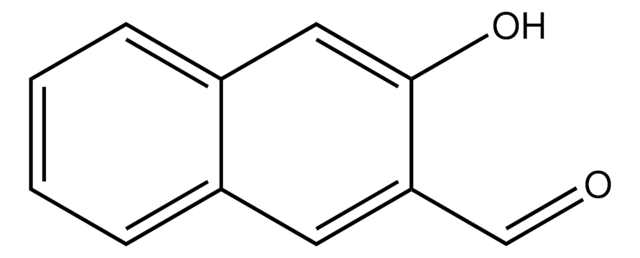
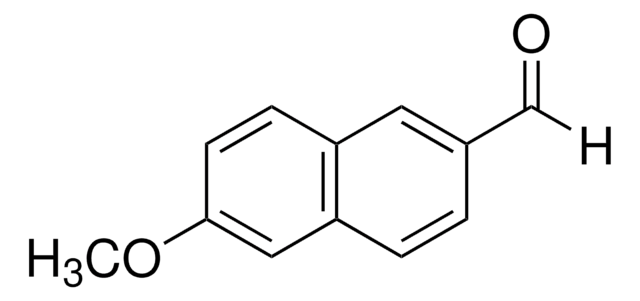
Linear Formula:
HOC10H7CHO
CAS Number:
Molecular Weight:
172.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
179-182 °C (lit.)
SMILES string
Oc1ccc(C=O)c2ccccc12
InChI
1S/C11H8O2/c12-7-8-5-6-11(13)10-4-2-1-3-9(8)10/h1-7,13H
InChI key
LORPDGZOLAPNHP-UHFFFAOYSA-N
Related Categories
Application
4-Hydroxy-1-naphthaldehyde was used in the preparation of racemic 1- and 2-naphthol analogues of tyrosine, 2-amino-3-(4-hydroxy-1-naphthyl)propanoic acid hydrochloride and 2-amino-3-(6-hydroxy-2-naphthyl)propanoic acid hydrobromide.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Syntheses of 1-and 2-naphthol analogs of DL-tyrosine. Potential fluorescent probes of peptide structure and dynamics in complex environments.
Vela MA, et al.
The Journal of Organic Chemistry, 55(9), 2913-2918 (1990)
Zi-Jian Chen et al.
Ecotoxicology and environmental safety, 196, 110533-110533 (2020-04-05)
1-naphthol (1-NAP) is the main metabolite of pesticide carbaryl and naphthalene, and is also a genotoxic and carcinogenic intermediate in the synthesis of organic compound, dyes, pigment and pharmaceutical industry. In this work, two novel haptens were designed and synthesized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service