All Photos(1)
About This Item
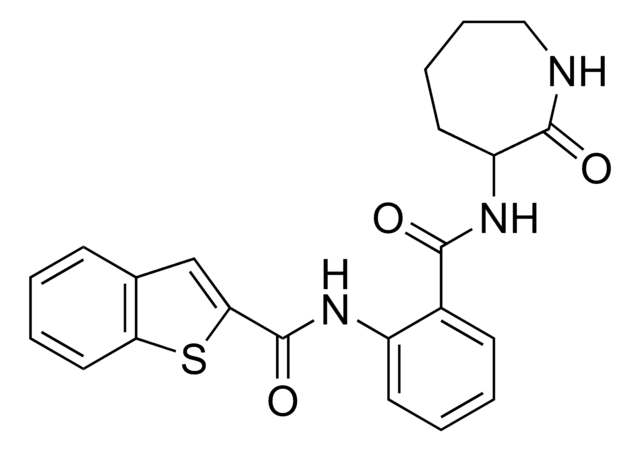
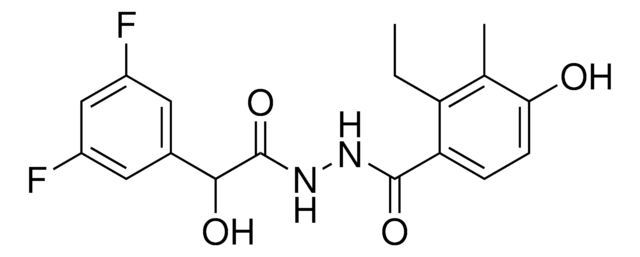
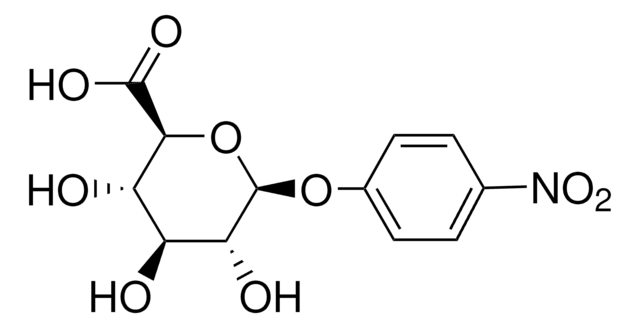
Empirical Formula (Hill Notation):
C15H10O4 · xH2O
CAS Number:
Molecular Weight:
254.24 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
storage condition
desiccated
solubility
DMSO: 24 mg/mL
storage temp.
room temp
SMILES string
Oc1ccc2C(=O)C=C(Oc2c1O)c3ccccc3
InChI
1S/C15H10O4/c16-11-7-6-10-12(17)8-13(19-15(10)14(11)18)9-4-2-1-3-5-9/h1-8,16,18H
InChI key
COCYGNDCWFKTMF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
7,8-Dihydroxyflavone hydrate has been used as tropomyosin-receptor-kinase B (TrkB) agonist in mice and to inhibit TrkB for monitoring evoked excitatory postsynaptic currents (eEPSCs).
Biochem/physiol Actions
7,8-Dihydroxyflavone (7,8-DHF) may be used to help identify and differentiate the physiological effects and cell signaling pathways mediated by TrkB activation, such as those involving, memory, vasorelaxation and hypertension. 7,8-DHF elicits protection in scopolamine induced Alzheimer-like pathologic dysfunction.
7,8-Dihydroxyflavone is a selective tyrosine kinase receptor B (TrkB) receptor agonist. It manifests all the therapeutic effects of brain-derived neurotrophic factor (BDNF)—such as protecting neurons from apoptosis, inhibiting kainic acid-induced toxicity, decreasing infarct volumes in stroke, and neuroprotecting in an animal model of Parkinson′s disease—without the poor pharmacokinetic profile of BDNF limiting its therapeutic potential.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiaojuan Liu et al.
Molecules (Basel, Switzerland), 25(4) (2020-02-23)
O-methylation of flavonoids is an important modification reaction that occurs in plants. O-methylation contributes to the structural diversity of flavonoids, which have several biological and pharmacological functions. In this study, an O-methyltransferase gene (CrOMT2) was isolated from the fruit peel
Vasorelaxing and antihypertensive effects of 7, 8-dihydroxyflavone
Huai R
American Journal of Hypertension, 27(5), 750-760 (2013)
Maria N Schultz et al.
Learning & memory (Cold Spring Harbor, N.Y.), 27(9), 346-354 (2020-08-21)
Angelman syndrome is a rare neurodevelopmental disorder caused by a mutation in the maternal allele of the gene Ube3a The primary symptoms of Angelman syndrome are severe cognitive deficits, impaired motor functions, and speech disabilities. Analogous phenotypes have been detected
Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem
Jang M, et al.
eLife, 8, e42156-e42156 (2019)
Autism-like behavior caused by deletion of vaccinia-related kinase 3 is improved by TrkB stimulation
Kang MS, et al.
The Journal of Experimental Medicine, 214(10), 2947-2966 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service