Y0000529
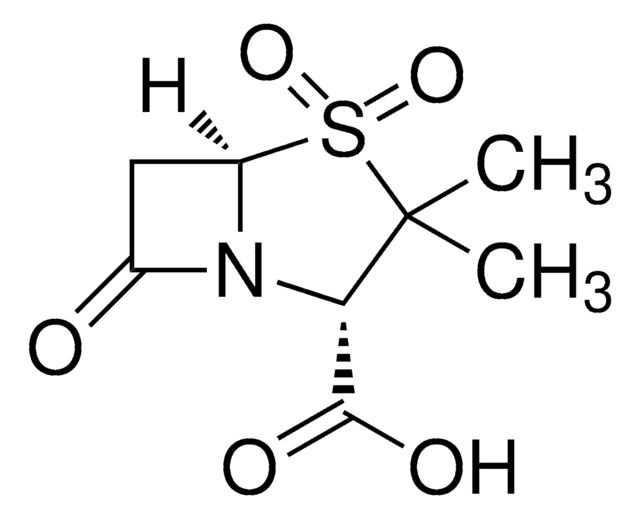
Sulbactam sodium
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
Sulbactam sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
sulbactam
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C8H11NO5S.Na/c1-8(2)6(7(11)12)9-4(10)3-5(9)15(8,13)14;/h5-6H,3H2,1-2H3,(H,11,12);/q;+1/p-1/t5-,6+;/m1./s1
InChI key
NKZMPZCWBSWAOX-IBTYICNHSA-M
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Sulbactam sodium EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Maura S de Oliveira et al.
Clinics (Sao Paulo, Brazil), 68(4), 569-573 (2013-06-20)
The objective of this study was to evaluate whether the outcomes of carbapenem-resistant Acinetobacter infections treated with ampicillin/sulbactam were associated with the in vitro susceptibility profiles. Twenty-two infections were treated with ampicillin/sulbactam. The median treatment duration was 14 days (range:
Alaa A Hassan et al.
Archiv der Pharmazie, 346(7), 562-570 (2013-06-19)
(E)-4-Aryl-2-[2-(1-substituted ethylidene)hydrazinyl]thiazoles and (Z)-3-substituted-4-aryl-2-[(E)-(1-phenylethylidene)hydrazono]-2,3-dihydrothiazoles were synthesized by the reaction of (substituted ethylidene)hydrazinecarbothioamides with ω-bromoacetophenones. The characterization of this new class of compounds was performed using different spectroscopic tools. The structure of (Z)-3-benzyl-4-(4-bromophenyl)-2-[(E)-(1-phenylethylidene)hydrazono]-2,3-dihydrothiazole 6e was unambiguously confirmed by single-crystal X-ray crystallography.
In vitro susceptibilities of clinical isolates of Escherichia coli and Klebsiella species to CSE1034 and other β-lactams.
Manu Chaudhary et al.
The Journal of antibiotics, 66(8), 495-497 (2013-04-25)
Mengtao Zhou et al.
Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.], 13(3), 212-215 (2013-05-31)
Our aim was to investigate the efficiency of continuous regional intra-arterial infusion (CRAI) with antisecretory agents and antibiotics in the treatment of infected pancreatic necrosis. CRAI was used as a new clinical technique to treat acute pancreatitis patients during a
Seyedali Seyedmajidi et al.
Arab journal of gastroenterology : the official publication of the Pan-Arab Association of Gastroenterology, 14(1), 1-5 (2013-04-30)
Selection of the best drug regimens for eradication of Helicobacter pylori infection especially in patients at risk of peptic ulcer relapses and the development of complications is challenging. This study assessed and compared the efficacy of the two common PPI
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service