T60607
Triethyl orthopropionate
97%
Synonym(s):
1,1,1-Triethoxypropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
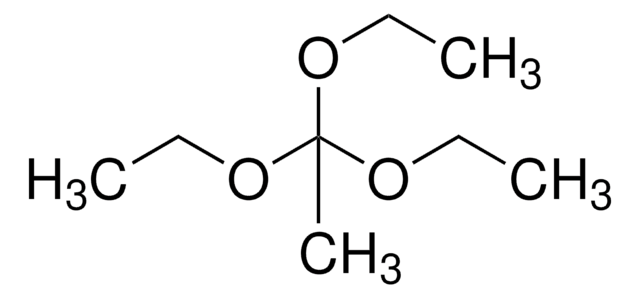
CH3CH2C(OC2H5)3
CAS Number:
Molecular Weight:
176.25
Beilstein:
906798
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.402 (lit.)
bp
155-160 °C (lit.)
density
0.876 g/mL at 25 °C (lit.)
SMILES string
CCOC(CC)(OCC)OCC
InChI
1S/C9H20O3/c1-5-9(10-6-2,11-7-3)12-8-4/h5-8H2,1-4H3
InChI key
FGWYWKIOMUZSQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Triethyl orthopropionate is commonly used in the Claisen rearrangement to construct new C-C bonds. It can react with pyrrole and chloroacetic acid to form 1,1,1- [tri-(pyrrol-2-yl)]propane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
140.0 °F - closed cup
Flash Point(C)
60 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Claisen rearrangement of (Z)-3-deoxy-3-C-[(hydroxymethyl) methylene]-1, 2: 5, 6-di-O-isopropylidene-. alpha.-D-ribo-hexofuranose with triethyl orthopropionate.
Tadano K, et al.
The Journal of Organic Chemistry, 54(5), 1223-1227 (1989)
Reactions between pyrrole and orthoesters: preparation of tri-(pyrrol-2-yl) alkanes.
Reese C B and Yan H
Tetrahedron Letters, 42(32), 5545-5547 (2001)
The Johnson-Claisen rearrangement of 3-hydroxy-2-methylenealkanenitriles: Stereoselective synthesis of functionalized trisubstituted alkenes.
Basavaiah D and Pandiaraju S
Tetrahedron Letters, 36(5), 757-758 (1995)
Stereoselectivity in the ortho ester Claisen rearrangements of the E and Z isomers of. gamma.-(1, 3-dioxan-4-yl) allyl alcohols.
Tadano K, et al.
The Journal of Organic Chemistry, 55(7), 2108-2113 (1990)
Acyclic stereoselection. 44. Diastereoselectivity in the ortho ester Claisen rearrangement of chiral propargylic alcohols. Use of. beta.-allenic esters as chiral methylmalonaldehyde synthons.
Henderson M A and Heathcock C H
The Journal of Organic Chemistry, 53(20), 4736-4745 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service