C94807
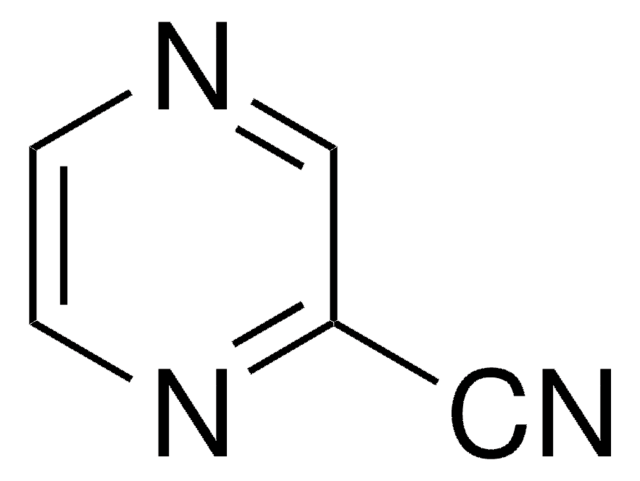
3-Pyridinecarbonitrile
98%
Synonym(s):
3-Cyanopyridine, Nicotinic acid nitrile, Nicotinonitrile
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H4N2
CAS Number:
Molecular Weight:
104.11
Beilstein:
107711
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
impurities
<2.5% acetone
bp
201 °C (lit.)
mp
48-52 °C (lit.)
SMILES string
N#Cc1cccnc1
InChI
1S/C6H4N2/c7-4-6-2-1-3-8-5-6/h1-3,5H
InChI key
GZPHSAQLYPIAIN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
190.4 °F
Flash Point(C)
88 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Li-Qun Jin et al.
New biotechnology, 28(6), 610-615 (2011-05-10)
2-Chloronicotinic acid is receiving much attention for its effective applications as a key precursor in the synthesis of pesticides and medicines. In this study, a strain ZJB-09149 converting 2-chloro-3-cyanopyridine to 2-chloronicotinic acid was newly isolated and identified as Rhodococcus erythropolis
Haiyang Fan et al.
Bioprocess and biosystems engineering, 40(8), 1271-1281 (2017-06-07)
A novel aliphatic nitrilase, REH16, was found in Ralstonia eutropha H16 and overexpressed in Escherichia coli BL21(DE3), and its enzymatic properties were studied. The temperature and pH optima were 37 °C and 6.6, respectively, and the best thermostability of the nitrilase
Anirban Banerjee et al.
Biotechnology and applied biochemistry, 37(Pt 3), 289-293 (2003-01-29)
A rapid, simple and sensitive fluorometric assay method for the determination of nitrilase activity is described. 3-Cyanopyridine was hydrolysed to nicotinic acid by Rhodococcus rhodochrous and the liberated NH(3) was allowed to react with buffered o -phthaldialdehyde-2-mercaptoethanol solution (pH 7.4)
H Adolfsson et al.
The Journal of organic chemistry, 65(25), 8651-8658 (2000-12-12)
The epoxidation of alkenes with 30% aqueous hydrogen peroxide is catalyzed efficiently by methyltrioxorhenium (MTO) in the presence of pyridine additives. The addition of 1-10 mol % of 3-cyanopyridine increases the system's efficiency for terminal and trans-disubstituted alkenes resulting in
Synthesis and antihistaminic activity of 2-guanadino-3-cyanopyridines and pyrido[2,3-d]-pyrimidines.
J M Quintela et al.
Bioorganic & medicinal chemistry, 5(8), 1543-1553 (1997-08-01)
2-Guanadino-3-cyanopyridines 8-33 and pyrido[2,3-d]-pyrimidines 35-52 were synthesized by nucleophilic displacement and cyclization of the chloroamidines 6a-d easily obtained by reaction of 2-aminocyanopyridines 5a-d with phosgene iminium chloride and their action on the release of histamine by mast cells examined under
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service