855286
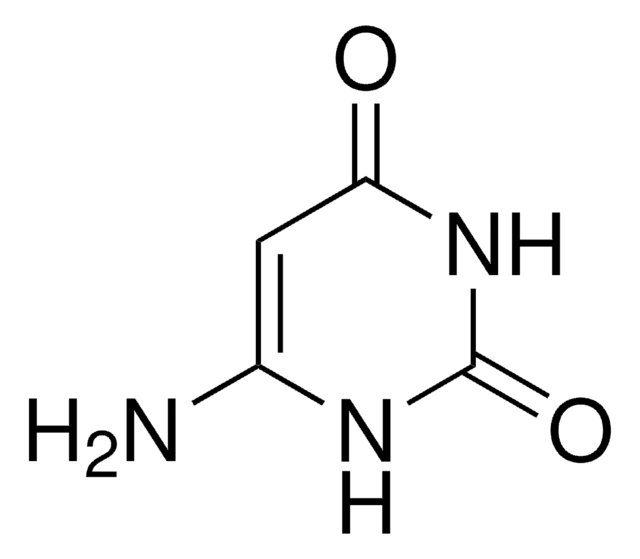
5-Aminouracil
98%
Synonym(s):
5-Amino-2,4-dihydroxypyrimidine, 5-Amino-2,4-pyrimidinediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5N3O2
CAS Number:
Molecular Weight:
127.10
Beilstein:
127250
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
>300 °C (lit.)
SMILES string
NC1=CNC(=O)NC1=O
InChI
1S/C4H5N3O2/c5-2-1-6-4(9)7-3(2)8/h1H,5H2,(H2,6,7,8,9)
InChI key
BISHACNKZIBDFM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Asmaa M Fahim et al.
Current computer-aided drug design, 16(4), 486-499 (2019-07-11)
In this investigation, 2-cyano-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl) acetamide (3) reacts with dimethylformamide dimethyl acetal (DMF-DMA) to afford the corresponding (E)- 2-cyano-3-(dimethylamino)-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylam-ide (4) utilizing microwave irradiation. The condensation reactions of acrylamide derivative 4 with hydrazine derivatives obtain pyrazole derivatives 6a and 6b; respectively. The
Del Campo et al.
Biology of the cell, 95(8), 521-526 (2003-11-25)
In the multinucleate cells induced in Allium cepa L. meristems, the nuclei surrounded by the largest cytoplasm environment complete replication earlier (advanced nuclei), but have a longer G2, than the others (delayed nuclei). Thus, all nuclei break down the nuclear
A González-Fernández et al.
Mutation research, 106(2), 255-264 (1982-12-01)
Chromosome damage induced in root meristems of Allium cepa L. by an 18-h treatment with 5-aminouracil (AU) was enhanced by 2-h pulses with 5 mM caffeine, the most effective pulse being given from the 8th to the 10th h after
Recovery from the 5-AU induced blockage in interphase: evidence for differential recovery.
D Davidson
Cytologia, 47(3-4), 545-553 (1982-12-01)
K K Upadhyay et al.
Organic & biomolecular chemistry, 8(21), 4892-4897 (2010-09-08)
A new fluorescent probe (5-[(4-diethylamino-2-hydroxy-benzylidene)-amino]-1H-pyrimidine-2, 4-dione) (Receptor 1) has been synthesized by the Schiff base condensation of 5-aminouracil with 4-(diethylamino)salicylaldehyde. The receptor 1 exhibits high selectively for Al(3+) in DMSO as well as in aqueous solution even in the presence
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service