722081
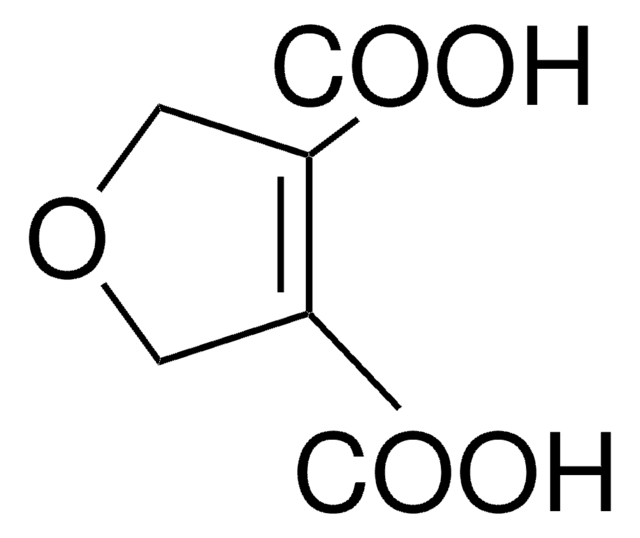
2,5-Furandicarboxylic acid
97%
Synonym(s):
Dehydromucic acid, FDCA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4O5
CAS Number:
Molecular Weight:
156.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
>300 °C
SMILES string
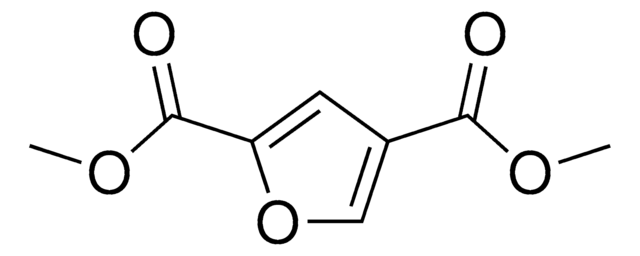
OC(=O)c1ccc(o1)C(O)=O
InChI
1S/C6H4O5/c7-5(8)3-1-2-4(11-3)6(9)10/h1-2H,(H,7,8)(H,9,10)
InChI key
CHTHALBTIRVDBM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,5-Furandicarboxylic acid (FDCA) is a renewable, greener substitute for terephthalate in the production of polyesters. It is widely used as a precursor for the synthesis of bio-based polyesters and various other polymers.
Applications of FDCA in the synthesis of several metal-organic frameworks (MOFs) have also been reported.
Applications of FDCA in the synthesis of several metal-organic frameworks (MOFs) have also been reported.
Other Notes
2,5-Furandicarboxylic acid has been included among Top 10 biorefinery carbohydrate derivatives for the production of biobased industrial products.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Giulia Guidotti et al.
International journal of molecular sciences, 20(9) (2019-05-06)
Biopolymers are gaining increasing importance as substitutes for plastics derived from fossil fuels, especially for packaging applications. In particular, furanoate-based polyesters appear as the most credible alternative due to their intriguing physic/mechanical and gas barrier properties. In this study, block
Niki Poulopoulou et al.
Polymers, 12(1) (2020-01-23)
Intending to expand the thermo-physical properties of bio-based polymers, furan-based thermoplastic polyesters were synthesized following the melt polycondensation method. The resulting polymers, namely, poly(ethylene 2,5-furandicarboxylate) (PEF), poly(propylene 2,5-furandicarboxylate) (PPF), poly(butylene 2,5-furandicarboxylate) (PBF) and poly(1,4-cyclohexanedimethylene 2,5-furandicarboxylate) (PCHDMF) are used in blends
The furan counterpart of poly (ethylene terephthalate): An alternative material based on renewable resources.
Gandini A, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 47(1), 295-298 (2009)
Crystalline Capsules: Metal?Organic Frameworks Locked by Size?Matching Ligand Bolts.
Wang H, et al.
Angewandte Chemie (International Edition in English), 54(20), 5966-5970 (2015)
Synthesis and properties of a bio-based epoxy resin from 2, 5-furandicarboxylic acid (FDCA).
Deng J, et al.
Royal Society of Chemistry Advances, 5(21), 15930-15939 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service