531413
2,2-Difluoro-2-(fluorosulfonyl)acetic acid
97%
Synonym(s):
(Fluorosulfonyl)difluoroacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FSO2CF2CO2H
CAS Number:
Molecular Weight:
178.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.36 (lit.)
bp
153 °C (lit.)
density
1.723 g/mL at 25 °C (lit.)
SMILES string
OC(=O)C(F)(F)S(F)(=O)=O
InChI
1S/C2HF3O4S/c3-2(4,1(6)7)10(5,8)9/h(H,6,7)
InChI key
VYDQUABHDFWIIX-UHFFFAOYSA-N
General description
2,2-Difluoro-2-(fluorosulfonyl)acetic acid reagent is employed as a difluorocarbene source for difluoromethylation of phenolic hydroxyl groups.
Application
2,2-Difluoro-2-(fluorosulfonyl)acetic acid may be used in the following processes:
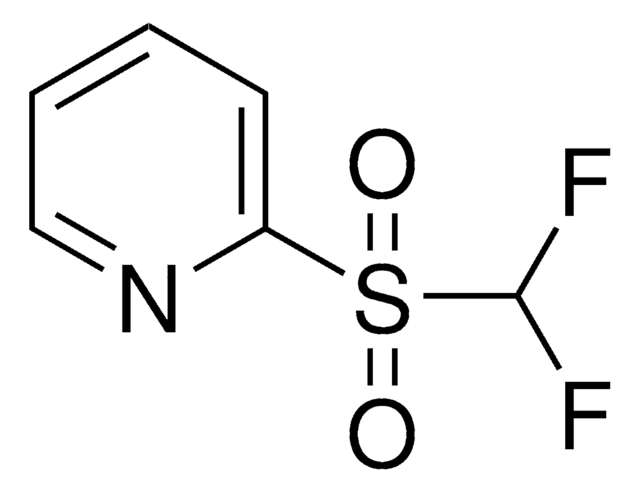
- Preparation of 1-difluoromethyl-2-oxo-1,2-dihydropyridine analogs by reacting with the corresponding 2-chloropyridines.
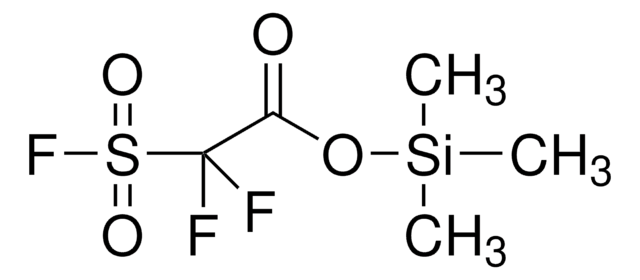
- Prepration of silyl fluorosulfonyldifluoroacetate as new highly efficient difluorocarbene reagent for cyclopropanation of alkenes.
- Regio- and stereoselective free radical fluoroalkylation of terminal alkenes and alkynes with iododifluoromethanesulfonamides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Skin Corr. 1A
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Free radical fluoroalkylation of terminal alkenes and alkynes with iododifluoromethanesulfonamides
Zhu, J. M.; et al.
Science China: Chemistry, 54, 95-102 (2011)
Trimethylsilyl fluorosulfonyldifluoroacetate (TFDA): a new, highly efficient difluorocarbene reagent
Dolbier, William R.; et al.
Journal of Fluorine Chemistry, 125, 459-469 (2004)
Free radical fluoroalkylation of terminal alkenes and alkynes with iododifluoromethanesulfonamides.
Zhu JM, et al.
Science China: Chemistry, 54(1), 95-102 (2011)
Preparation and use of a new difluorocarbene reagent
Dolbier, W. R., Jr.; et al.
Organic Syntheses, 80, 172-176 (2003)
Makoto Ando et al.
Organic letters, 8(17), 3805-3808 (2006-08-11)
[reaction: see text] A novel one-pot synthesis of N-difluoromethyl-2-pyridones is described. N-(Pyridin-2-yl)acetamide derivatives were excellent precursors for the preparation of N-difluoromethyl-2-pyridone derivatives. Difluoromethylation of 2-acetaminopyridine derivatives was achieved with sodium chlorodifluoroacetate as a difluorocarbene source in the presence of a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![4-(Acetylamino)phenyl]imidodisulfuryl difluoride ≥98%](/deepweb/assets/sigmaaldrich/product/structures/101/806/3f40354f-e903-4ea0-9654-10872377816c/640/3f40354f-e903-4ea0-9654-10872377816c.png)


![Zinc bis[bis(trimethylsilyl)amide] 97%](/deepweb/assets/sigmaaldrich/product/structures/294/819/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d/640/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d.png)