All Photos(1)
About This Item
Empirical Formula (Hill Notation):
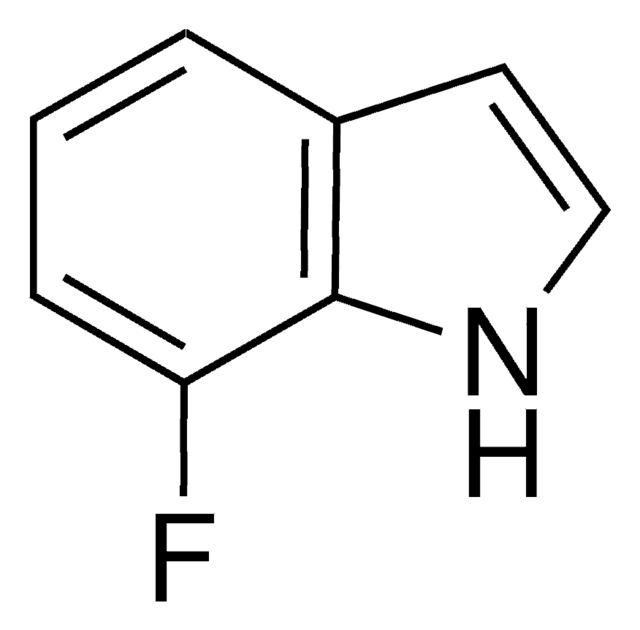
C8H6FN
CAS Number:
Molecular Weight:
135.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
bp
90 °C/0.4 mmHg (lit.)
mp
30-32 °C (lit.)
SMILES string
Fc1cccc2[nH]ccc12
InChI
1S/C8H6FN/c9-7-2-1-3-8-6(7)4-5-10-8/h1-5,10H
InChI key
ZWKIJOPJWWZLDI-UHFFFAOYSA-N
Application
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Reactant for preparation of antifungal agents
- Reactant for preparation of Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the Management of Hyperglycemia in Diabetes
- Reactant for preparation of Potent Selective Serotonin Reuptake Inhibitors
- Reactant for preparation of Inhibitors of HIV-1 attachment
- Reactant for preparation of monoamine reuptake inhibitors
- Reactant for preparation of histone deacetylase (HDAC) inhibitors
- Reactant for preparation of inhibitors of proliferation of human breast cancer cells
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
(Erratum)
Journal of Medicinal Chemistry, 2442-2442 null
Synthesis of (L)-4-Fluorotryptophan.
Konas DW, et al.
Synthetic Communications, 42(1), 144-152 (2012)
Journal of the Chemical Society. Perkin Transactions 1, 1765-1765 (1994)
J L Malleron et al.
Journal of medicinal chemistry, 36(9), 1194-1202 (1993-04-30)
A series of new indole derivatives (2-28) has been prepared in the search for novel 5-HT uptake inhibitors. These compounds were obtained by the condensation of N-(chloroalkyl) naphthalenesultam derivatives with the appropriate amine in presence of a base, at reflux
A synthesis of (-)-indolactam V.
Semmelhack MF and Rhee H.
Tetrahedron Letters, 34(39), 1395-1398 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service