159549
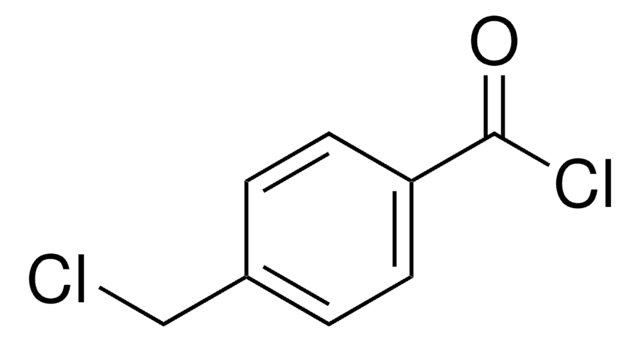
4-(Bromomethyl)benzoic acid
97%
Synonym(s):
α-Bromo-p-toluic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2C6H4CO2H
CAS Number:
Molecular Weight:
215.04
Beilstein:
1862870
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
224-229 °C (lit.)
SMILES string
OC(=O)c1ccc(CBr)cc1
InChI
1S/C8H7BrO2/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4H,5H2,(H,10,11)
InChI key
CQQSQBRPAJSTFB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-(Bromomethyl)benzoic acid was used in the chemical modification of 5,10,15,20-tetra(m-hydroxyphenyl)chlorin (temoporfin), second generation photosensitizer. It was also used in the synthesis of 4-(5-arylidene-2,4-dioxothiazolidin-3-yl) methylbenzoic acids.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chendong Ji et al.
Chemistry, an Asian journal, 11(16), 2316-2321 (2016-07-14)
Electrospun ultrathin fiber-based sensors are desirable because of their practicality and sensitivity. Ammonia-detection systems are in high demand in different areas, including the industrial and agricultural fields. However, current technologies rely on large and complex instruments that restrict their actual
Q Yu et al.
Chemical communications (Cambridge, England), 50(81), 12150-12153 (2014-09-02)
LiYF4:Tm(3+)/Yb(3+) upconverting nanoparticles (UCNPs) were functionalized with the second generation photosensitizer 5,10,15,20-tetra(m-hydroxyphenyl)chlorin (m-THPC, Temoporfin, Foscan®). m-THPC was modified using 4-(bromomethyl)benzoic acid, which induced a bathochromic shift of the m-THPC blue absorption peak. The nanoconstruct causes up to 70% cell death
Marcela S Lopes et al.
European journal of medicinal chemistry, 46(11), 5443-5447 (2011-09-24)
A series of nitroaromatic compounds was synthesized and evaluated as potential antileishmanial and trypanocidal agents. Five compounds exerted significant anti-leishmanial activity in vitro against promastigotes forms of Leishmania (L.) amazonensis, with IC(50) in the range of 23-59 μmol L(-1), but
Rosanna Maccari et al.
Bioorganic & medicinal chemistry, 15(15), 5137-5149 (2007-06-05)
4-(5-Arylidene-2,4-dioxothiazolidin-3-yl)methylbenzoic acids (2) were synthesized and evaluated in vitro as inhibitors of PTP1B and LMW-PTP, two protein tyrosine phosphatases (PTPs) which act as negative regulators of the metabolic and mitotic signalling of insulin. The synthesis of compounds 2 represents an
Zhen Wu et al.
Chemistry, an Asian journal, 11(21), 3102-3106 (2016-09-21)
Spiropyran (SP), which can respond to acid, ultraviolet irradiation, and mechanical force, has received great attention as a classic molecule for the construction of stimuli-responsive materials. However, the self-assembly behavior of SP with a tunable morphology has rarely been investigated.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service