102261
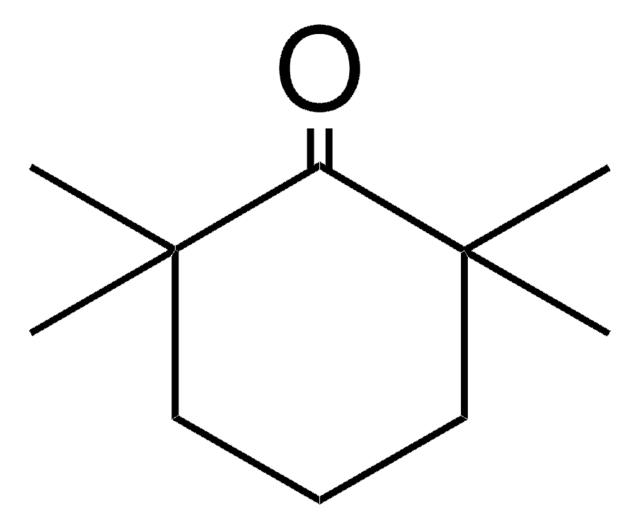
2,6-Dimethylcyclohexanone, mixture of isomers
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C6H8(=O)
CAS Number:
Molecular Weight:
126.20
Beilstein:
1099000
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.447 (lit.)
bp
174-176 °C (lit.)
density
0.925 g/mL at 25 °C (lit.)
SMILES string
CC1CCCC(C)C1=O
InChI
1S/C8H14O/c1-6-4-3-5-7(2)8(6)9/h6-7H,3-5H2,1-2H3
InChI key
AILVYPLQKCQNJC-UHFFFAOYSA-N
General description
2,6-Dimethylcyclohexanone, mixture of isomers is a clear, light yellow liquid. The adsorption of traces of 2,6-dimethylcyclohexanone, a volatile organic compound, from liquid toluene was studied. cis- and trans-isomers of 2,6-Dimethylcyclohexanonereacts with hydroxylamine hydrochloride and potassium hydroxide in methanol solution gives cis- and trans-dimethylcyclohexanone oxime .
Application
2,6-Dimethylcyclohexanone was used in tetraoxane synthesis and in evaluation of their anti-malarial activity.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
123.8 °F - closed cup
Flash Point(C)
51 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Adsorption of Volatile Organic Compounds. Experimental and Theoretical Study.
Brunchi CC, et al.
Industrial & Engineering Chemistry Research, 51(51), 16697-16708 (2012)
K J McCullough et al.
Journal of medicinal chemistry, 43(6), 1246-1249 (2000-03-29)
Two tetramethyl-substituted dispiro-1,2,4,5-tetraoxanes (7,8,15, 16-tetraoxadispiro[5.2.5.2]hexadecanes) 3 and 4 were designed as metabolically stable analogues of the dimethyl-substituted dispiro-1, 2,4,5-tetraoxane prototype WR 148999 (2). For a positive control we selected the sterically unhindered tetraoxane 5 (7,8,15, 16-tetraoxadispiro[5.2.5.2]hexadecane), devoid of any substituents.
Photochemistry of Oximes III. The Photochemical Beckmann Rearrangement.
Cunningham M, et al.
Canadian Journal of Chemistry, 49(17), 2891-2896 (1971)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service