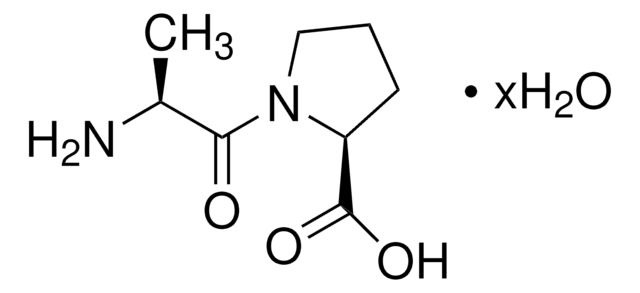
P0880
Pro-Gly
≥98% (TLC), suitable for cell culture
Synonym(s):
L-prolyl-glycine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H12N2O3
CAS Number:
Molecular Weight:
172.18
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Pro-Gly,
Assay
≥98% (TLC)
form
powder
technique(s)
cell culture | mammalian: suitable
color
white
application(s)
cell analysis
storage temp.
−20°C
SMILES string
OC(=O)CNC(=O)[C@@H]1CCCN1
InChI
1S/C7H12N2O3/c10-6(11)4-9-7(12)5-2-1-3-8-5/h5,8H,1-4H2,(H,9,12)(H,10,11)/t5-/m0/s1
InChI key
RNKSNIBMTUYWSH-YFKPBYRVSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Biochem/physiol Actions
PRO-GLY is a dipeptide that has previously been shown to prevent progression of diabetes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T A Gudasheva et al.
European journal of drug metabolism and pharmacokinetics, 22(3), 245-252 (1997-07-01)
The metabolism of a new piracetam analogue, the dipeptide cognitive enhancer N-phenylacetyl-L-prolylglycine ethyl ester (GVS-111) was studied in vivo. GVS-111 itself was not found in rat brain 1 h after 5 mg/kg i.p. administration up to limit of detection (LOD)
I P Ashmarin et al.
Biochemistry. Biokhimiia, 63(2), 119-124 (1998-06-02)
Our own data and data from the literature on the regulatory role of the simplest proline-containing peptides GP, PG, PGP, GPGG, and cyclic-PG are summarized. These peptides are involved in homeostasis of gastric mucosa and the anticoagulant and fibrinolytic potential
Z Dzakula et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 135(2), 454-465 (1999-01-08)
Analytical expressions have been derived that translate uncertainties in distance constraints (obtained from NMR investigations) into uncertainties in atom positions in the maximum likelihood (ML) structure consistent with these inputs. As a test of this approach, a comparison was made
Feng-Chun Wu et al.
The Journal of organic chemistry, 74(13), 4812-4818 (2009-05-23)
Tetrapeptides, containing a terminated primary amine and conformationally restricted D-Pro-Gly or D-Pro-Aib (2-aminoisobutanoic acid) segment as a strongly beta-turn-nucleating element, were designed and synthesized with condensation of N-module dipeptides with C-module dipeptides in solution. They were first applied to catalyze
Shigeo Hayakawa et al.
Journal of the American Chemical Society, 129(25), 7936-7949 (2007-06-07)
We report a combined experimental and computational study of the proline effect in model dipeptides Pro-Gly and Gly-Pro. Gas-phase protonated peptide ions were discharged by glancing collisions with potassium or cesium atoms at 3 keV collision energies, and the peptide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service