T35955
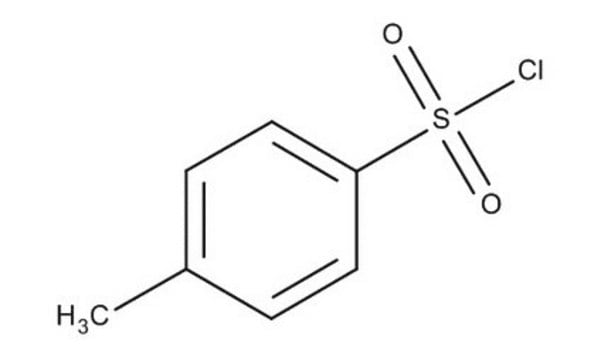
p-Toluenesulfonyl chloride
reagent grade, ≥98%
Synonym(s):
Tosyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
CH3C6H4SO2Cl
CAS Number:
Molecular Weight:
190.65
Beilstein:
607898
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
reagent grade
Quality Level
vapor pressure
1 mmHg ( 88 °C)
Assay
≥98%
bp
134 °C/10 mmHg (lit.)
mp
65-69 °C (lit.)
SMILES string
Cc1ccc(cc1)S(Cl)(=O)=O
InChI
1S/C7H7ClO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3
InChI key
YYROPELSRYBVMQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
p-toluenesulfonyl chloride may be used along with N-methylimidazole for the esterification or thioesterification of carboxylic acids and alcohols or thiols. It may also be used as a chlorine source for α-chlorination of ketones in the presence of lithium diisopropylamide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
262.4 °F - closed cup
Flash Point(C)
128 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rince Wong et al.
The Journal of organic chemistry, 72(10), 3969-3971 (2007-04-21)
A facile and general protocol for the preparation of isothiocyanates from alkyl and aryl amines is reported. This method relies on a tosyl chloride mediated decomposition of a dithiocarbamate salt that is generated in situ by treatment of an amine
Y Tabata et al.
Biomaterials, 7(3), 234-238 (1986-05-01)
The cyanogen bromide (CNBr) activation method was adopted to link collagen molecules onto the surface of cellulose and poly(vinyl alcohol) (PVA) films via covalent bonding. The amount of bound protein was determined by the ninhydrin method and found to be
I M Kung et al.
Biomaterials, 16(8), 649-655 (1995-05-01)
Three different surface modifications were conducted on the membranes of double-layer alginate/poly(L-lysine) microcapsules with tosyl chloride-activated poly(ethylene glycol), cyanuric chloride-activated poly(ethylene glycol) and tosyl chloride-activated poly(vinyl alcohol), separately. All these surface modifications strengthen the microcapsular membranes. Of the three surface-modified
Katarzyna Dziarkowska et al.
Analytica chimica acta, 606(2), 184-193 (2007-12-18)
Determination of polyamines in biological fluids possesses medical diagnostic relevance. Despite the vast panel of analytical methods developed for polyamines they are not applied in routine clinical usage, mainly due to the time and labor consuming sample preparation step and
Salvatore Lombardo et al.
Langmuir : the ACS journal of surfaces and colloids, 33(22), 5473-5481 (2017-05-13)
The interaction of bovine serum albumin (BSA) with sulfated, carboxylated, and pyridinium-grafted cellulose nanocrystals (CNCs) was studied as a function of the degree of substitution by determining the adsorption isotherm and by directly measuring the thermodynamics of interaction. The adsorption
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service