700023P
Avanti
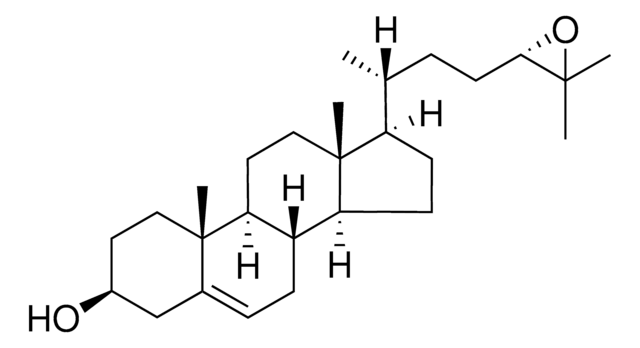
7α,27-dihydroxy-4-cholesten-3-one
Avanti Research™ - A Croda Brand
Synonym(s):
Cholest-4-en-3-one, 7,26-dihydroxy-, (7α,25R)-
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C27H44O3
CAS Number:
Molecular Weight:
416.64
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 1 mg (700023P-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand
shipped in
dry ice
storage temp.
−20°C
General description
7α,27-dihydroxy-4-cholesten-3-one synthesized by the hydroxylation of 27-hydroxycholesterol in the presence of the enzyme cholesterol 7 α-hydroxylase (CYP7A1) and is catabolized to bile acid. 7α,27-dihydroxy-4-cholesten-3-one is present majorly in fibroblasts and its conversion from cholesterol occurs in extrahepatic tissues.
Application
7α,27-dihydroxy-4-cholesten-3-one may be used as a ligand to test its effect on Epstein-Barr virus-induced molecule 2 (EB12) activation in guanosine 5′-O-(3-thio)triphosphate ([35S] GTPγS) binding assay.
Biochem/physiol Actions
7α,27-dihydroxy-4-cholesten-3-one is a suppressor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.
Packaging
5 mL Amber Glass Screw Cap Vial (700023P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hepatic and extrahepatic dehydrogenation/isomerization of 5-cholestene-3beta, 7alpha-diol: localization of 3beta-hydroxy-Delta5-C27-steroid dehydrogenase in pig tissues and subcellular fractions
Furster C
Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1436(3), 343-353 (1999)
27-Hydroxylated Low Density Lipoprotein (LDL) Cholesterol Can Be Converted to 7alpha, 27-Dihydroxy-4-cholesten-3-one (Cytosterone) before Suppressing Cholesterol Production in Normal Human Fibroblasts EVIDENCE THAT AN ALTERED METABOLISM OF LDL CHOLESTEROL
Axelson M and Larsson O
The Journal of Biological Chemistry, 271(22), 12724-12736 (1996)
Identification of structural motifs critical for epstein-barr virus-induced molecule 2 function and homology modeling of the ligand docking site
Zhang L, et al.
Molecular Pharmacology, 82(6), 1094-1103 (2012)
On the substrate specificity of human CYP27A1: implications for bile acid and cholestanol formation.
Maria Norlin et al.
Journal of lipid research, 44(8), 1515-1522 (2003-06-05)
The mitochondrial sterol 27-hydroxylase (CYP27A1) is required for degradation of the C27-sterol side chain in bile acid biosynthesis. CYP27A1 seems, however, to have roles beyond this, as illustrated by patients with a deficient sterol 27-hydroxylase due to mutations of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service