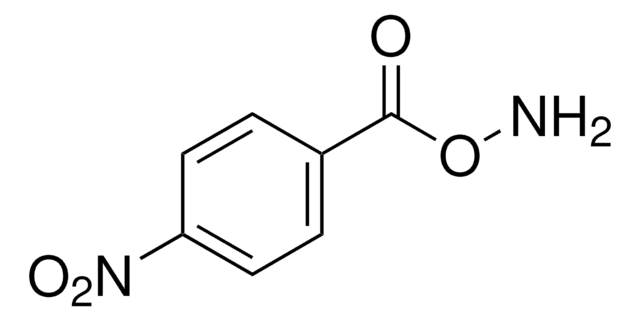
B22984
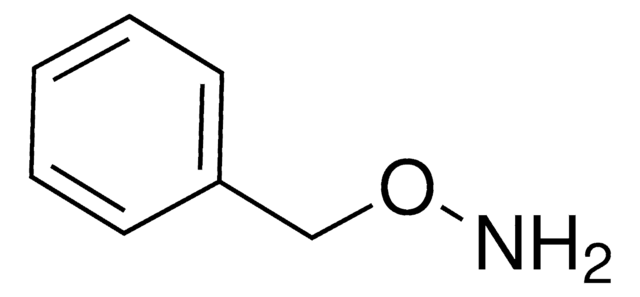
O-Benzylhydroxylamine hydrochloride
99%
Synonym(s):
Benzyloxyamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2ONH2 · HCl
CAS Number:
Molecular Weight:
159.61
Beilstein:
3687991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
238 °C (subl.) (lit.)
SMILES string
Cl.NOCc1ccccc1
InChI
1S/C7H9NO.ClH/c8-9-6-7-4-2-1-3-5-7;/h1-5H,6,8H2;1H
InChI key
HYDZPXNVHXJHBG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Effective reagent used to prepare α-hydroxybenzylamines from α-hydroxyketones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A A Purmal et al.
Mutation research, 364(3), 193-207 (1996-12-02)
Duplex oligonucleotides containing the base lesion analogs, O-methylhydroxylamine- and O-benzylhydroxylamine-modified abasic (AP) sites, were substrates for the DNA N-glycosylases endonuclease III, formamidopyrimidine DNA N-glycosylase and T4 endonuclease V. These N-glycosylases are known to have associated AP lyase activities. In contrast
S M Breckenridge et al.
Journal of chromatography. B, Biomedical sciences and applications, 694(2), 289-296 (1997-07-04)
Extraction and derivatization of carbonyls to benzyloximes, pentafluorobenzyloximes or 2,4-dinitrophenylhydrazones is simplified and reaction times are substantially reduced by simultaneous sorption and derivatization from aqueous solution onto a solid phase. In this reaction a macroreticular polystyrene-divinylbenzene resin acts as a
G Cardillo et al.
Organic letters, 3(8), 1165-1167 (2001-05-12)
[reaction: see text]. The 1,4-addition of O-benzylhydroxylamine to alpha,beta-unsaturated imide 1 in the presence of BF3.Et2O proceeds with the preferential attack of the nucleophile on the Cbeta-re face. To explain this unexpected reactivity 1H, 13C, and 11B NMR investigations have
Han Fu et al.
Journal of combinatorial chemistry, 9(5), 804-810 (2007-08-11)
A highly regioselective and traceless solid-phase route to 1,7,8-trisubstituted purines has been developed. This methodology could be extended to the preparation of 8-azapurines and [i]-condensed purines. A representative set of 17 purines, azapurines, and [i]-condensed purines was synthesized. This paper
[Thermographic analysis of precipitates formed by the interaction of active ingredients and additives].
G Stampf et al.
Acta pharmaceutica Hungarica, 53(6), 268-272 (1983-11-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service