87212
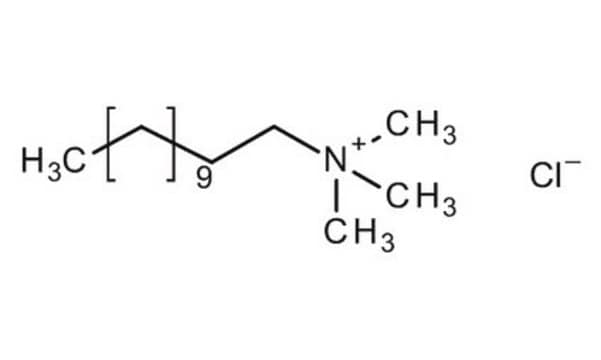
Trimethyl-tetradecylammonium chloride
≥98.0% (AT)
Synonym(s):
Tetradecyltrimethylammonium chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)13N(Cl)(CH3)3
CAS Number:
Molecular Weight:
291.94
Beilstein:
3916572
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (AT)
form
powder
mp
~250 °C (dec.)
SMILES string
[Cl-].CCCCCCCCCCCCCC[N+](C)(C)C
InChI
1S/C17H38N.ClH/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18(2,3)4;/h5-17H2,1-4H3;1H/q+1;/p-1
InChI key
CEYYIKYYFSTQRU-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Keisuke Matsuoka et al.
Journal of colloid and interface science, 333(2), 641-645 (2009-02-27)
The maximum solubilities of homologous series of n-alkylbenzene and n-perfluoroalkylbenzene in aqueous solutions of surfactants n-tetradecyltrimethylammonium chloride (TTAC) and N-(1,1-dihydroperfluorodecyl)-N,N,N-trimethylammonium chloride (C10F-TAC) were measured as a function of the surfactant concentration at 298.2 K. There are four solubilization systems in
Cexiong Fu et al.
Journal of chromatography. A, 1216(10), 1901-1907 (2009-02-03)
The usefulness of the micellar selectivity triangle (MST) for prediction and interpretation of separation patterns in micellar electrokinetic chromatography (MEKC) separations is presented. In addition, we demonstrate the capability of controlling selectivity properties of micelles through addition of organic modifiers
Aline Delbos et al.
Physical review. E, Statistical, nonlinear, and soft matter physics, 84(1 Pt 1), 011404-011404 (2011-08-27)
We investigate experimentally the behavior of liquid foams pumped at a given flow rate through a single pore, in the situation where the pore diameter is smaller than the bubble diameter. Results reveal that foam invasion can be observed only
Irma Orentaitė et al.
Electrophoresis, 32(5), 604-613 (2011-02-04)
For tetradecyltrimethylammonium bromide in boric acid/borate or acetic acid/acetate buffer and NaCl or CaCl₂ as the added salt, it is investigated whether the retention behaviour of weak acids in MEKC with cationic surfactant can be modelled by assuming for the
Yuri Park et al.
Journal of colloid and interface science, 360(2), 440-456 (2011-05-24)
Organoclays were synthesised through ion exchange of a single surfactant for sodium ions, and characterised by a range of method including X-ray diffraction (XRD), BET, X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FT-IR), and transmission electron
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service