All Photos(1)
About This Item
Linear Formula:
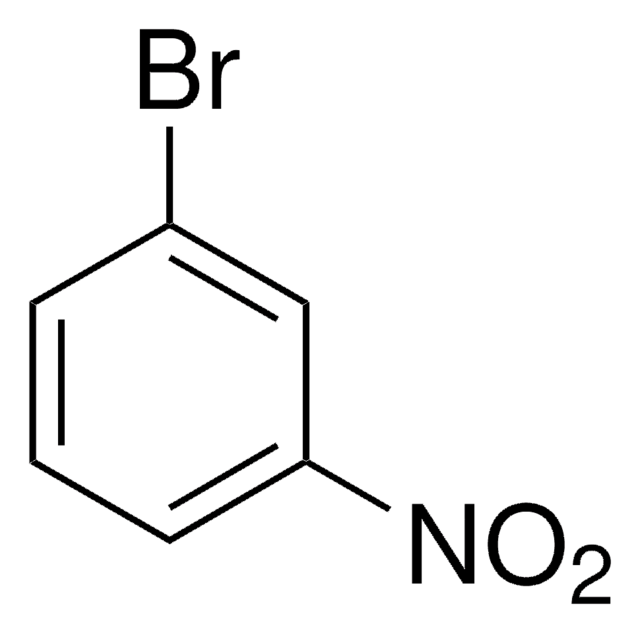
BrC6H3(NO2)2
CAS Number:
Molecular Weight:
247.00
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
71-73 °C (lit.)
functional group
bromo
SMILES string
[O-][N+](=O)c1ccc(Br)c(c1)[N+]([O-])=O
InChI
1S/C6H3BrN2O4/c7-5-2-1-4(8(10)11)3-6(5)9(12)13/h1-3H
InChI key
PBOPJYORIDJAFE-UHFFFAOYSA-N
Related Categories
Application
1-Bromo-2,4-dinitrobenzene has been used:
- in the preparation of 2,4-dinitrophenol via treatment with KO2-crown ether complex in benzene
- as substrate in protein determination and glutathione S-transferase (GST) assay of chicken and rat prostaglandin D2 synthase (PGDS)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The reaction of poly-L-ornithine with 1-halo-2,4-dinitrobenzenes.
R C Parker et al.
The International journal of biochemistry, 13(4), 513-516 (1981-01-01)
A M Thomson et al.
The Biochemical journal, 333 ( Pt 2), 317-325 (1998-07-11)
The Expressed Sequence Tag database has been screened for cDNA clones encoding prostaglandin D2 synthases (PGDSs) by using a BLAST search with the N-terminal amino acid sequence of rat GSH-dependent PGDS, a class Sigma glutathione S-transferase (GST). This resulted in
Nucleophilic radical aromatic substituion with superoxide ion.
Frimer A and Rosenthal I.
Tetrahedron Letters, 17(32), 2809-2812 (1976)
K C Chen et al.
Drug and chemical toxicology, 3(3), 305-318 (1980-01-01)
The formation of glutathione (GSH) conjugate in the detoxification of [Ring-UL-14C]-2,4-dinitrobromobenzene (DNBB) was investigated using rat liver cytosolic fraction. The mercapturic acid conjugate in rats was also studied by collection of urine of rats dosed with radioactive DNBB by intraperitoneal
R L Gupta et al.
Mutation research, 381(1), 41-47 (1997-12-24)
The 1-halogen substituted 2,4-dinitrobenzenes have been found to be mutagenic in Salmonella TA98 with an activity order of 1-fluoro > 1-chloro > 1-bromo > 1-iodo. This specific activity was not lowered in the nitroreductase deficient strain TA98NR and O-acetyltransferase deficient
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service