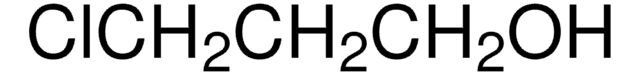189316
3-Chloro-2,2-dimethyl-1-propanol
99%
Synonym(s):
2-Chloromethyl-2-methyl-1-propanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCH2C(CH3)2CH2OH
CAS Number:
Molecular Weight:
122.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
refractive index
n20/D 1.45 (lit.)
bp
87 °C/35 mmHg (lit.)
mp
34-36 °C (lit.)
functional group
chloro
hydroxyl
SMILES string
CC(C)(CO)CCl
InChI
1S/C5H11ClO/c1-5(2,3-6)4-7/h7H,3-4H2,1-2H3
InChI key
CAZPRAORHCOIHC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Chloro-2,2-dimethyl-1-propanol undergoes oxidation with pyridinium chlorochromate to yield 3-chloro-2,2-dimethylpropanal which spontaneously trimerizes to s-trioxane.
Application
3-Chloro-2,2-dimethyl-1-propanol has been used in combinatorial preparation of new aroma-impact compounds such as polyfunctional thiols.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
159.8 °F - closed cup
Flash Point(C)
71 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2, 4, 6-Tri (2'-chloro-1', 1'-dimethylethyl)-s-trioxane: Synthesis, Spectroscopy and Crystal Structure.
Tarbutton GL and Valente EJ.
Journal of Chemical Crystallography, 40(2), 126-129 (2010)
Catherine Vermeulen et al.
Combinatorial chemistry & high throughput screening, 9(8), 583-590 (2006-10-05)
Combinatorial chemistry was shown to be an efficient tool for the preparation of new aroma-impact compounds. In this case, polyfunctional thiols were synthesized quickly using halide reagents or Bunte salt intermediates. They were separated by gas chromatography and then characterized
Xin Min et al.
Royal Society open science, 5(5), 180156-180156 (2018-06-13)
A novel alkyl lithium-based initiator with relatively large steric hindrance, tert-butyldimethylsiloxydimethylpropyl lithium (TBDMSODPrLi), was designed and synthesized. By using TBDMSODPrLi, hydroxyl-terminated polybutadiene (HTPB) was prepared via anionic polymerization. The macromolecular structure of HTPB was characterized and verified by FTIR and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service