SML0531
BIO-Acetoxime
≥98% (HPLC)
Synonym(s):
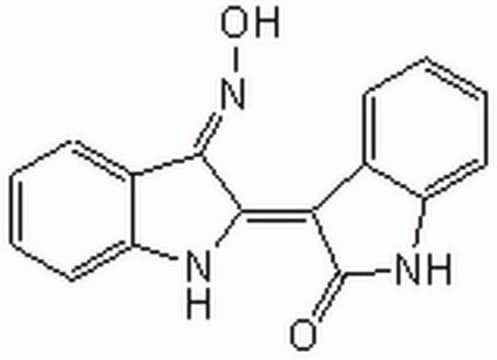
(2′Z,3′E)-6-Bromoindirubin-3′-acetoxime, (3Z)-3-[(3E)-3-[(Acetyloxy)imino]-1,3-dihydro-2H-indol-2-ylidene]-6-bromo-1,3-dihydro-2H-Indol-2-one, 6-Bromoindirubin acetoxime, 6-Bromoindirubin-3′-acetoxime, BIA
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
, dark red to purple
solubility
H2O: 2 mg/mL (clear solution, warmed)
storage temp.
2-8°C
SMILES string
CC(=O)O\N=C1\C(Nc2ccccc12)=C3\C(=O)Nc4cc(Br)ccc34
InChI
1S/C18H12BrN3O3/c1-9(23)25-22-16-12-4-2-3-5-13(12)20-17(16)15-11-7-6-10(19)8-14(11)21-18(15)24/h2-8,20H,1H3,(H,21,24)/b17-15-,22-16+
InChI key
MENDIXKFTXYTHJ-HPBPSMLHSA-N
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Glycogen synthase kinase 3 (GSK-3) is a highly conserved family of serine/threonine kinases for over 100 proteins in many pathways. See a list of accepted GSK-3 modulators and related products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service