79417
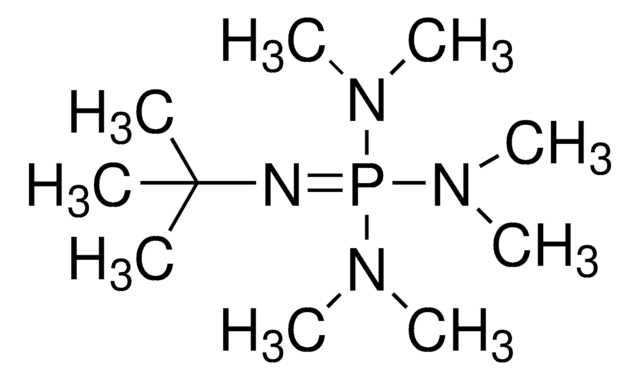
Phosphazene base P2-Et
≥98.0% (NT)
Synonym(s):
1-Ethyl-2,2,4,4,4-pentakis(dimethylamino)-2λ5,4λ5-catenadi(phosphazene), Tetramethyl(tris(dimethylamino)phosphoranylidene)phosphorictriamid-Et-imin
About This Item
Recommended Products
Assay
≥98.0% (NT)
form
liquid
refractive index
n20/D 1.492 (lit.)
n20/D 1.492
bp
96 °C/0.05 mmHg (lit.)
density
1.02 g/mL at 25 °C (lit.)
SMILES string
CCN=P(N=P(N(C)C)(N(C)C)N(C)C)(N(C)C)N(C)C
InChI
1S/C12H35N7P2/c1-12-13-20(15(2)3,16(4)5)14-21(17(6)7,18(8)9)19(10)11/h12H2,1-11H3
InChI key
CFUKEHPEQCSIOM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
This strong non-ionic base has the ability to catalyze a wide variety of organic transformations such as:
- Palladium-catalyzed cross-coupling reactions when used in combination with tBuXPhos or tBuBrettPhos G3 precatalysts.
- Deprotonation of ortho-halobenzyl sulfones to generate α-sulfonyl benzylic carbanions.
- Conversion of vinyl sufone to allyl sulfone.
- α-Alkylation of 2-phenyl-2-oxazoline-4-carbonylcamphorsultam in the presence of tetrabutylammonium bromide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Phosphazene base reagents are available as monomeric (P1 and BEMP), dimeric (P2), and tetrameric (P4) bases with different side chains to control their sterical hindrance.
Related Content
The Nicewicz lab is focused on the discovery of new and powerful reaction methodologies that proceed via the intermediacy of highly reactive cation radical species. Included in these transformations are anti-Markovnikov selective additions of amines, alcohols, carboxylic acids, amides and mineral acids to alkenes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

![Tetrakis[tris(dimethylamino)phosphoranylidenamino]phosphonium chloride 95%](/deepweb/assets/sigmaaldrich/product/structures/160/963/9dd6d457-17b2-44dc-8ea2-d3c0475b3664/640/9dd6d457-17b2-44dc-8ea2-d3c0475b3664.png)