791369
Tang-Yu Auxiliary
97%
Synonym(s):
2-(2-Cyanophenoxy)-2-fluoroacetic acid
About This Item
Recommended Products
Assay
97%
form
solid
reaction suitability
reaction type: C-C Bond Formation
reagent type: catalyst
reagent type: ligand
reaction type: C-H Activation
mp
105-108 °C
SMILES string
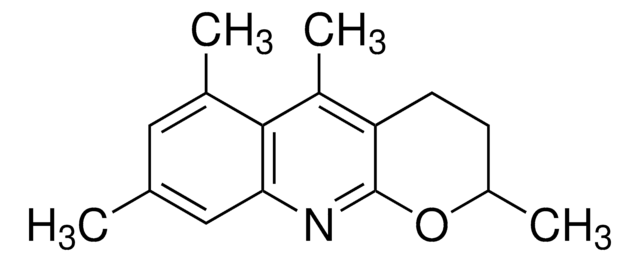
O=C(O)C(F)OC1=C(C#N)C=CC=C1
InChI
1S/C9H6FNO3/c10-8(9(12)13)14-7-4-2-1-3-6(7)5-11/h1-4,8H,(H,12,13)
InChI key
WPPDDNKKUDYVMF-UHFFFAOYSA-N
Related Categories
Application
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Yu program centers around the discovery of catalytic carbon–carbon and carbon–heteroatom bond forming reactions based on C–H activation. Target transformations are selected to enable 1) the use of simple and abundant starting materials such as aliphatic acids, amines and alcohols, and 2) disconnections that drastically shorten the synthesis of a drug molecule or a major class of biologically active compounds.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)