All Photos(1)
About This Item
Empirical Formula (Hill Notation):
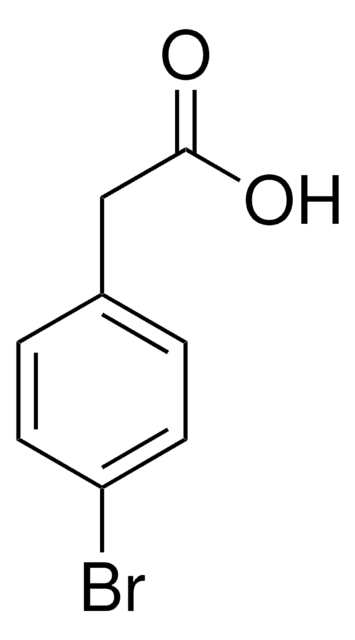
C9H7NO2
CAS Number:
Molecular Weight:
161.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
150-154 °C (lit.)
SMILES string
OC(=O)Cc1ccc(cc1)C#N
InChI
1S/C9H7NO2/c10-6-8-3-1-7(2-4-8)5-9(11)12/h1-4H,5H2,(H,11,12)
InChI key
WEBXRQONNWEETE-UHFFFAOYSA-N
Application
4-Cyanophenylacetic acid can be used as a nitrile precursor to synthesize 1,2,4,5-tetrazines by reacting with aliphatic nitriles and hydrazine in the presence of Lewis acid metal catalysts.
It can also be used as a reactant to prepare:
It can also be used as a reactant to prepare:
- 4-pyrrolo[1,2-a]quinoxalin-4-ylbenzonitrile by copper-catalyzed reaction with 1-(2-aminoaryl)pyrrole in the presence of 2,2′-bipyridyl as the ligand.
- 4-(1,2-Diphenyl-1H-imidazol-4-yl)benzonitrile by one-pot three-component reaction with N-phenylbenzamidine and nitromethane via activation of C-H and N-H bonds.
- 4-Cyano-N,N-di-2-propen-1-ylbenzeneacetamide by reacting with diallylamine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An Efficient Synthesis of Pyrrolo [1, 2-a] quinoxalines by Copper-Catalyzed C- H Activation of Arylacetic Acids
Lade JJ, et al.
Asian Journal of Organic Chemistry, 6(11), 1579-1583 (2017)
Copper-Catalyzed Simultaneous Activation of C-H and N-H Bonds: Three-Component One-Pot Cascade Synthesis of Multisubstituted Imidazoles
Pardeshi SD, et al.
Synthesis, 50(02), 361-370 (2018)
Flow synthesis of cyclobutanones via [2+ 2] cycloaddition of keteneiminium salts and ethylene gas
Battilocchio C, et al.
Reaction Chemistry & Engineering, 2(3), 295-298 (2017)
Metal-catalyzed one-pot synthesis of tetrazines directly from aliphatic nitriles and hydrazine.
Jun Yang et al.
Angewandte Chemie (International ed. in English), 51(21), 5222-5225 (2012-04-19)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

