All Photos(1)
About This Item
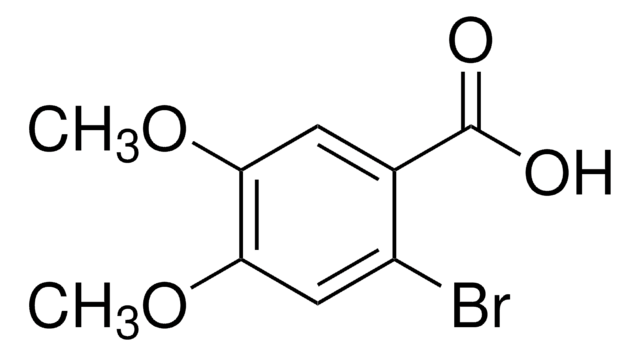
Linear Formula:
C6H3Cl(I)COOH
CAS Number:
Molecular Weight:
282.46
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
163-167 °C (lit.)
SMILES string
OC(=O)c1ccc(Cl)cc1I
InChI
1S/C7H4ClIO2/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3H,(H,10,11)
InChI key
LRRDANNSUCQNDU-UHFFFAOYSA-N
Related Categories
General description
4-Chloro-2-iodobenzoic acid is an electron-deficient substituted 2-iodobenzoic acid. It reacts with ynamide to provide the 3,4-disubstituted isocoumarin.
Application
4-Chloro-2-iodobenzoic acid may be used to synthesize:
- 6-chloro-3-(4-methoxyphenyl)-1H-isochromen-1-one
- (Z)-3-benzylidene-5-chloroisobenzofuran-1(3H)-one
- 6-chloro-3-pentyl-1H-isochromen-1-one
- 4-chloro-2-iodobenzophenone
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Regioselective synthesis of 3, 4-disubstituted isocoumarins through the Pd-catalyzed annulation of 2-iodoaromatic acids with ynamides.
Liu H, et al.
Chemical Communications (Cambridge, England), 52(41), 6801-6804 (2016)
Veronika Hampl et al.
Scientia pharmaceutica, 79(1), 21-30 (2011-05-28)
New isocoumarins were prepared in an efficient way from 2-iodobenzoic acid derivatives and hept-1-yne in a Sonogashira reaction, followed by spontaneous cyclization. Catalytic hydrogenation gave the corresponding dihydroisocoumarins. A 4-chloroisocoumarin was prepared on an alternative pathway. Some of the new
The synthesis and electronic absorption spectra of 3-phenyl-3 (4-pyrrolidino-2-substituted phenyl)-3H-naphtho [2, 1-b] pyrans: further exploration of the ortho substituent effect.
Gabbutt CD, et al.
Tetrahedron, 62(4), 737-745 (2006)
Regioselective One-Pot Synthesis of Isocoumarins and Phthalides from 2-Iodobenzoic Acids and Alkynes by Temperature Control.
Kumar MR, et al.
Advanced Synthesis & Catalysis, 355(16), 3221-3230 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service