All Photos(1)
About This Item
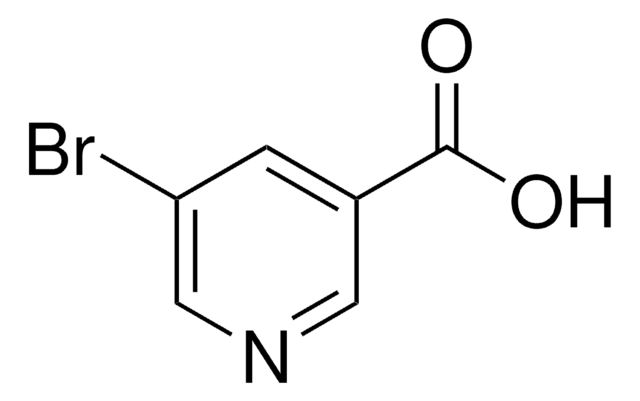
Empirical Formula (Hill Notation):
C5H3BrN4O
CAS Number:
Molecular Weight:
215.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
>350 °C (lit.)
SMILES string
BrC1=Nc2nc[nH]c2C(=O)N1
InChI
1S/C5H3BrN4O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H2,7,8,9,10,11)
InChI key
ONXCBJOMYNPZNI-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sheng Ding et al.
Journal of combinatorial chemistry, 4(2), 183-186 (2002-03-12)
A resin-capture and release strategy for making combinatorial 2,6,9-trisubstituted purine libraries is demonstrated by capturing N9-derivatized purines at the C6 position with a thio-modified polymer. The C2 fluoro group is subsequently substituted with primary and secondary amines followed by thioether
R S Sodum et al.
Chemical research in toxicology, 11(12), 1453-1459 (1998-12-22)
2-Nitropropane, an industrial chemical and a hepatocarcinogen in rats, induces aryl sulfotransferase-mediated liver DNA and RNA base modifications [Sodum, R. S., Sohn, O. S., Nie, G., and Fiala, E. S. (1994) Chem. Res. Toxicol. 7, 344-351]. Two of these modifications
John M Caddell et al.
The Journal of organic chemistry, 69(9), 3212-3215 (2004-04-24)
A convergent synthesis of adenosine A2a agonist 1 in the form of its maleate salt 2 was achieved. The key step in this approach was the highly selective 9beta-glycosylation reaction between 2-haloadenines or an N(2)-alkyl-6-chloroguanine and a D-ribose derivative containing
M M Butler et al.
Nucleic acids research, 18(24), 7381-7387 (1990-12-25)
6-(p-Hydroxyphenylhydrazino)uracil (H2-HPUra) is a selective and potent inhibitor of the replication-specific class III DNA polymerase (pol III) of Gr+ bacteria. Although formally a pyrimidine, H2-HPUra derives its inhibitory activity from its specific capacity to mimic the purine nucleotide, dGTP. We
Andrzej Manikowski et al.
Journal of medicinal chemistry, 48(11), 3919-3929 (2005-05-27)
Derivatives of the herpes simplex thymidine kinase inhibitor HBPG [2-phenylamino-9-(4-hydroxybutyl)-6-oxopurine] have been synthesized and tested for inhibitory activity against recombinant enzymes (TK) from herpes simplex types 1 and 2 (HSV-1, HSV-2). The compounds inhibited phosphorylation of [3H]thymidine by both enzymes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service