All Photos(2)
About This Item
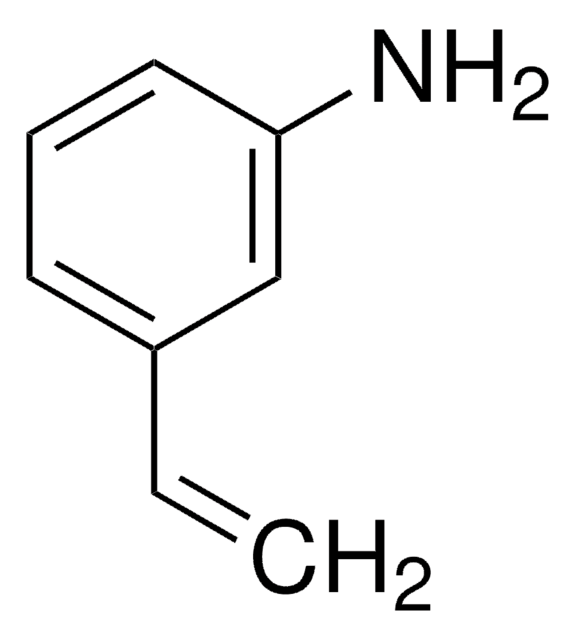
Linear Formula:
HC≡CC6H4NH2
CAS Number:
Molecular Weight:
117.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
reaction suitability
reaction type: click chemistry
storage temp.
2-8°C
SMILES string
Nc1cccc(c1)C#C
InChI
1S/C8H7N/c1-2-7-4-3-5-8(9)6-7/h1,3-6H,9H2
InChI key
NNKQLUVBPJEUOR-UHFFFAOYSA-N
General description
3-Ethynylaniline is a terminal alkyne that can be prepared by the reduction of 3-ethylnylnitrobenzene.
Application
3-Ethynylaniline may be used in the synthesis of the following benzoxazine monomers:
- bis{4-[3-(3-ethynylphenyl)(2H,4Hbenzo[3,4-e]1,3-oxazin-6-yloxy)]phenyl}phenylphosphino-1-one
- [3-(3-ethynylphenyl)(2H,4Hbenzo[3,4-e]1,3-oxazaperhydroin-6-yl)][3-(3-ethynylphenyl)(2H,4H-benzo[3,4-e]1,3-oxazin-6-yl)]phenylphosphino-1-one
- bis[3-(3-ethynylphenyl)(2H,4Hbenzo[3,4-e]1,3-oxazin-6-yl)oxy]phenylphosphino-1-one
- To synthesize succin(m-ethynyl)dianilide and sebaco(m-ethynyl)dianilide.
- As a reagent in the multi-step synthesis of erlotinib hydrochloride.
- To prepare 3-(3-ethynylphenyl)-6-methyl-2H, 4H-benzo[e]1,3-oxazine.
- To prepare 3-[3-(4-acetylamino-benzyl)-[1,2,4]oxadiazol-5- yl]-N-(3-ethynyl-phenyl)-propionamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Side-chain type benzoxazine-functional cellulose via click chemistry.
Agag T, et al.
Journal of Applied Polymer Science, 125(2), 1346-1351 (2012)
Paul L Chariou et al.
Nature nanotechnology, 14(7), 712-718 (2019-05-22)
Large doses of chemical pesticides are required to achieve effective concentrations in the rhizosphere, which results in the accumulation of harmful residues. Precision farming is needed to improve the efficacy of pesticides, but also to avoid environmental pollution, and slow-release
Qilin Mei et al.
Polymers, 12(5) (2020-05-03)
Benzoxazine resin has been paid more attention in the fields of aviation, electronics, automobiles and new energy industries because of its excellent comprehensive performance. Further application is limited, however, by shortcomings such as high brittleness and high curing temperature. Furthermore
Synthesis, analgesic and anti-inflammatory activities of novel 3-(4-acetamido-benzyl)-5-substituted-1,2,4-oxadiazoles.
Farooqui M, et al.
European Journal of Medicinal Chemistry, 44(2), 794-799 (2009)
Laura Marín-Caba et al.
Langmuir : the ACS journal of surfaces and colloids, 35(1), 203-211 (2018-12-24)
The design of versatile tools to improve cell targeting and drug delivery in medicine has become increasingly pertinent to nanobiotechnology. Biological and inorganic nanocarrier drug delivery systems are being explored, showing advantages and disadvantages in terms of cell targeting and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![[3R(1′R,4R)]-(+)-4-Acetoxy-3-[1-(tert-butyldimethylsilyloxy)ethyl]-2-azetidinone 98%](/deepweb/assets/sigmaaldrich/product/structures/346/391/4981e055-bdcd-454c-8c8f-519083b64769/640/4981e055-bdcd-454c-8c8f-519083b64769.png)







![2-[(Trimethylsilyl)ethynyl]aniline 97%](/deepweb/assets/sigmaaldrich/product/structures/194/066/182b08e4-35d7-4b7b-8958-c917f64391fc/640/182b08e4-35d7-4b7b-8958-c917f64391fc.png)

![4-[(Trimethylsilyl)ethynyl]aniline 96%](/deepweb/assets/sigmaaldrich/product/structures/322/881/781cb36d-38c8-406f-afb2-1a4488f884e2/640/781cb36d-38c8-406f-afb2-1a4488f884e2.png)