All Photos(2)
About This Item
Empirical Formula (Hill Notation):
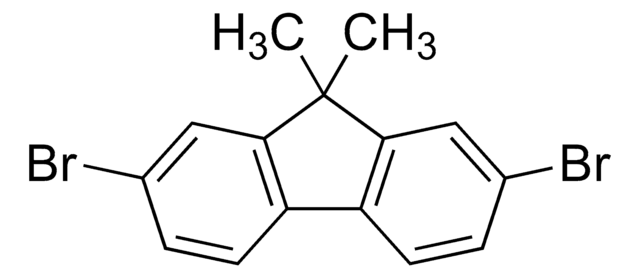
C13H8Br2
CAS Number:
Molecular Weight:
324.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
164-166 °C (lit.)
SMILES string
Brc1ccc-2c(Cc3cc(Br)ccc-23)c1
InChI
1S/C13H8Br2/c14-10-1-3-12-8(6-10)5-9-7-11(15)2-4-13(9)12/h1-4,6-7H,5H2
InChI key
AVXFJPFSWLMKSG-UHFFFAOYSA-N
Related Categories
General description
2,7-Dibromofluorene is a halogenated polycyclic aromatic compound and its vapour pressure has been measured using the Knudsen effusion method.
Application
2,7-Dibromofluorene was used as a template for the N-carbazole capped oligofluorenes which show potential as hole-transporting materials for organic light emitting devices (OLEDs). It may be used in the synthesis of conjugated polymer, poly[9,9′-bis(6′′-N,N,N-trimethylammonium)hexyl)fluorene-co-alt-4,7-(2,1,3-benzothiadiazole) dibromide] (PFBT), used in label-free DNA microarrays. It was used in the preparartion of blue photoluminescent unsymmetrically substituted polyfluorene. It was also used in the preparation of 2,7-dibromofluorene monomers containing benzyl ether dendrons (generations 1, 2 and 3) in the 9,9′-position of the fluorene ring, such as:
- 9,9-bis[(3,5-bis(benzyloxy)benzyloxy)methyl]-2,7-dibromofluorene
- 9,9-bis[(3,5-bis(3,5-bis(benzyloxy)benzyloxy)benzyloxy)-methyl] 2,7-dibromo-fluorene
- 9,9-bis[(3,5-bis(3,5-bis(3,5-bis(benzyloxy)benzyloxy)benzyloxy) benzyloxy)-methyl]-2,7-dibromofluorene
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H Z Tang et al.
Chemical communications (Cambridge, England), (23)(23), 2426-2427 (2002-09-25)
The first unsymmetrically substituted polyfluorene bearing a bulky poly(benzyl ether) dendron and less bulky 3,6-dioxaoctyl groups in the 9-position was designed and synthesized, which gives almost a pure bluish photoluminescence with negligible weak greenish excimer emission around 520 nm even
D Marsitzky et al.
Journal of the American Chemical Society, 123(29), 6965-6972 (2001-07-19)
The synthesis and characterization of complex dendritic, rigid rod poly-2,7-fluorene homopolymers and copolymers via a macromonomer approach is reported. Several 2,7-dibromofluorene monomers containing benzyl ether dendrons (generations 1, 2, and 3) in the 9,9'-position of the fluorene ring were prepared
Tetrahedron Letters, 48, 89-89 (2007)
Jinxia Fu et al.
Environmental toxicology and chemistry, 31(3), 486-493 (2011-12-06)
Knowledge of vapor pressure of organic pollutants is essential in predicting their fate and transport in the environment. In the present study, the vapor pressures of 12 halogenated polycyclic aromatic compounds (PACs), 9-chlorofluorene, 2,7-dichlorofluorene, 2-bromofluorene, 9-bromofluorene, 2,7-dibromofluorene, 2-bromoanthracene, 9-chlorophenanthrene, 9-bromophenanthrene
Bin Liu et al.
Nature protocols, 1(4), 1698-1702 (2007-05-10)
We describe the synthesis of poly[9,9'-bis(6''-N,N,N-trimethylammonium)hexyl)fluorene-co-alt-4,7-(2,1,3-benzothiadiazole) dibromide] (PFBT), a cationic, water-soluble conjugated polymer used in label-free DNA microarrays. This polymer was designed to have a maximum absorbance of close to 488 nm, which meets the excitation wavelength of most commercial
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)