All Photos(2)
About This Item
Empirical Formula (Hill Notation):
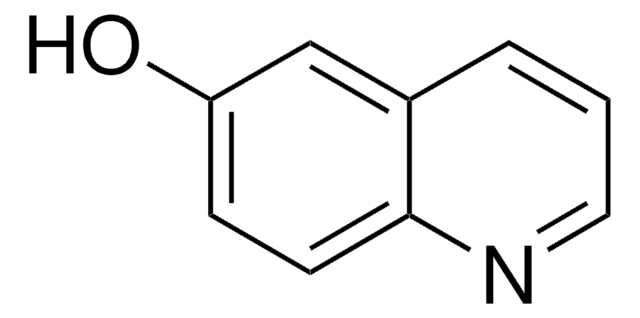
C9H7NO
CAS Number:
Molecular Weight:
145.16
Beilstein:
114514
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
223-226 °C (lit.)
SMILES string
Oc1cccc2ncccc12
InChI
1S/C9H7NO/c11-9-5-1-4-8-7(9)3-2-6-10-8/h1-6,11H
InChI key
GYESAYHWISMZOK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Quinolinol (5-Hydroxyquinoline) was used as an internal standard in the reaction to measure morphine concentration in serum or plasma. It was also used as a lipophilic chelator and it decreased the rate of deoxygenation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Hollstein et al.
Journal of the National Cancer Institute, 60(2), 405-410 (1978-02-01)
Quinoline, a hepatocarcinogen in rats, and 23 quinoline derivatives were tested for mutagenic activity with the Ames Salmonella typhimurium assay. Quinoline, 5-hydroxyquinoline, and 8-hydroxyquinoline were mutagenic in strain TA 100 when Aroclor 1254-induced rat (male outbred Sprague-Dawley) liver homogenate was
Analysis of morphine in serum by high performance liquid chromatography with amperometric detection.
H Vandenberghe et al.
Therapeutic drug monitoring, 4(3), 307-314 (1982-01-01)
In this report we describe a rapid and sensitive micromethod using high performance liquid chromatography (HPLC) with electrochemical detection (ED) to measure morphine concentration in serum or plasma. The separation of morphine and the internal standard 5-hydroxyquinoline, from interfering compounds
R Deslauriers et al.
Biochimica et biophysica acta, 886(3), 319-326 (1986-05-29)
A spectrophotometric assay has been devised to measure oxygen consumption non-invasively in intact murine red cells parasitized by Plasmodium berghei. The method uses oxyhemoglobin in the erythrocytes both as a source of oxygen and as an indicator of oxygen consumption.
Markus Brinkmann et al.
Chemical research in toxicology, 32(4), 698-707 (2019-03-22)
Hydroxylation of polyaromatic compounds through cytochromes P450 (CYPs) is known to result in potentially estrogenic transformation products. Recently, there has been an increasing awareness of the importance of alternative pathways such as aldehyde oxidases (AOX) or N-methyltransferases (NMT) in bioactivation
Nihal Kuş et al.
The journal of physical chemistry. A, 119(24), 6296-6308 (2015-05-31)
The structure, infrared spectrum, and photochemistry of 5-hydroxyquinoline (5HQ) were studied by matrix isolation infrared spectroscopy, complemented by theoretical calculations performed at the DFT(B3LYP)/6-311++G(d,p) level of approximation. According to the calculations, the trans conformer of 5HQ (with the OH group
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service