CRM04195
Dichlofluanid
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
product line
TraceCERT®
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
storage temp.
2-8°C
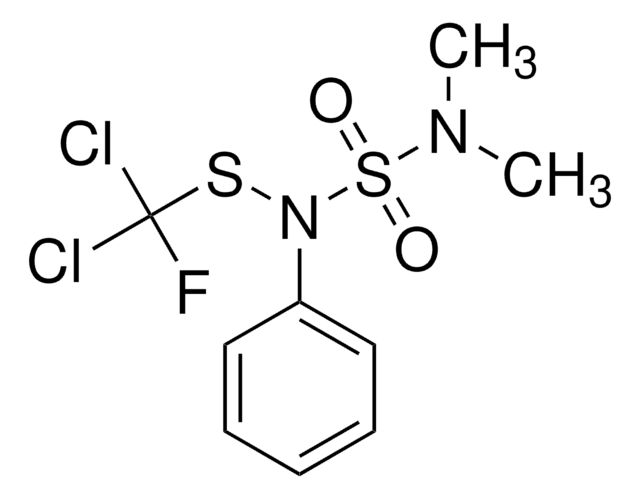
SMILES string
CN(C)S(=O)(=O)N(SC(F)(Cl)Cl)c1ccccc1
InChI
1S/C9H11Cl2FN2O2S2/c1-13(2)18(15,16)14(17-9(10,11)12)8-6-4-3-5-7-8/h3-7H,1-2H3
InChI key
WURGXGVFSMYFCG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Dichlofluanid is a booster biocide that belongs to the sulfamide class. It binds with thiol groups, including the tripeptide glutathione, to inhibit mitochondrial respiration and lipid peroxidation.
Dichlofluanid is widely used in agriculture to control scab, brown rot, and storage diseases in apples and pears, Botrytis spp., Alternaria spp., Clasterosporium spp., downy mildew, etc. in vines, pome fruit, stone fruit, berries, strawberries, hops, tomatoes, cucurbits, vegetables, ornamentals, and conifer seedbeds.
Dichlofluanid is not approved in the European Union. A default MRL of 0.01 mg/kg has been set according to Art 18(1)(b) Reg. 396/2005.
Application
- To determine the concentration of dichlofluanid and its metabolite in seawater and sediment of three marinas in Greece before and during the yachting season
- To evaluate the biochemical and physiological effects of dichlofluanid exposure on the brown mussel Perna perna
- Photocatalytic degradation of chlorothalonil and dichlofluanid over TiO2 suspensions
- Testing toxicity of the fungicides penconazol, cymoxanil, and dichlofluanid and their effects on the specific growth rate, biomass production, respiration rate, and yeast viability
- To estimate the rates of disappearance of dichlofluanid and tebuconazole following successive applications to two varieties of lettuce grown in greenhouse conditions
Recommended products
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Acute 1 - Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service