R7635
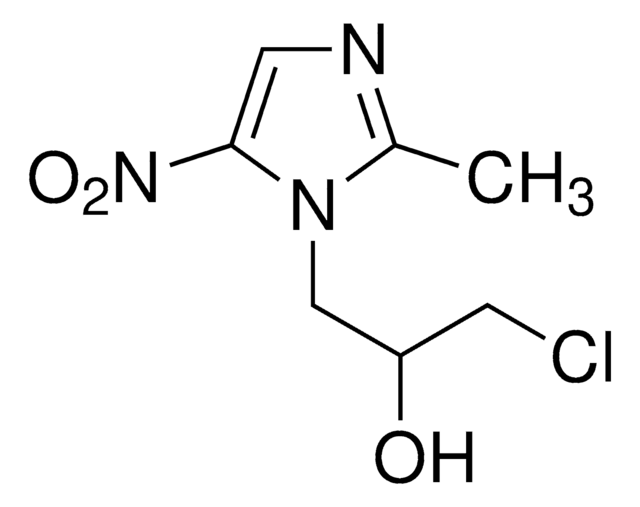
Ronidazole
analytical standard
Synonym(s):
1-Methyl-2-(carbamoyloxymethyl)-5-nitroimidazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8N4O4
CAS Number:
Molecular Weight:
200.15
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥95%
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
forensics and toxicology
pharmaceutical (small molecule)
veterinary
format
neat
storage temp.
−20°C
SMILES string
Cn1c(COC(N)=O)ncc1N(=O)=O
InChI
1S/C6H8N4O4/c1-9-4(3-14-6(7)11)8-2-5(9)10(12)13/h2H,3H2,1H3,(H2,7,11)
InChI key
PQFRTXSWDXZRRS-UHFFFAOYSA-N
General description
Ronidazole is a nitroimidazole compound, commonly used as antiprotozoal and antibacterial agent.
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Ronidazole may be used as a reference standard in the determination of the analyte in poultry muscle and eggs using high-performance liquid chromatography with ultraviolet detection and confirmatory analysis by atmospheric pressure chemical ionization-mass spectrometry.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and antiprotozoal activity of 1-(3-chloro-2-hydroxypropyl)-substituted nitroimidazoles.
Hoffer Max and Grunberg E
Journal of Medicinal Chemistry, 17(9), 1019-1020 (1974)
Elizabeth J Kather et al.
Journal of veterinary internal medicine, 21(5), 966-970 (2007-10-18)
The nitroimidazole, ronidazole, has been demonstrated to have in vitro and in vivo activity against the protozoan Tritrichomonas foetus in cats. The purpose of this study was to evaluate the in vitro susceptibility of feline T. foetus isolates obtained from
Neurotoxicosis in 4 cats receiving ronidazole.
Terri W Rosado et al.
Journal of veterinary internal medicine, 21(2), 328-331 (2007-04-13)
Determination of dimetridazole, ronidazole and their common metabolite in poultry muscle and eggs by high performance liquid chromatography with UV detection and confirmatory analysis by atmospheric pressure chemical ionisation mass spectrometry.
Sams JM, et al.
Analyst, 123(1-2), 2545-2549 (1998)
J L Gookin et al.
Journal of veterinary internal medicine, 24(4), 1003-1007 (2010-05-25)
The mainstays of treatment for clinically important trichomonad infections are the 5-nitroimidazoles. Metronidazole resistance of feline Tritrichomonas foetus is presumed because of common treatment failure, and tinidazole does not consistently eradicate infection. To date, ronidazole is the only drug demonstrated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service