N26806
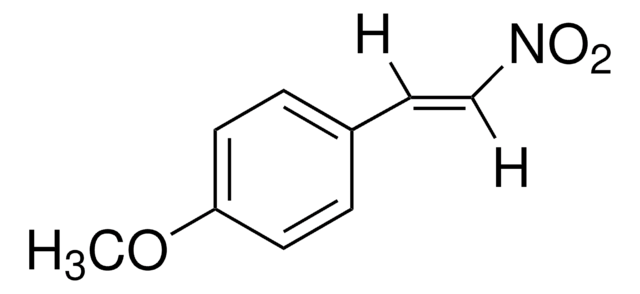
trans-β-Nitrostyrene
99%
Synonym(s):
trans-beta-Nitrostyrene, trans-1-Nitro-2-phenylethylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=CHNO2
CAS Number:
Molecular Weight:
149.15
Beilstein:
1210066
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
bp
250-260 °C (lit.)
mp
55-58 °C (lit.)
storage temp.
2-8°C
SMILES string
[O-][N+](=O)\C=C\c1ccccc1
InChI
1S/C8H7NO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H/b7-6+
InChI key
PIAOLBVUVDXHHL-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bridging between organocatalysis and biocatalysis: asymmetric addition of acetaldehyde to β-nitrostyrenes catalyzed by a promiscuous proline-based tautomerase.
Ellen Zandvoort et al.
Angewandte Chemie (International ed. in English), 51(5), 1240-1243 (2011-12-23)
Yufeng Miao et al.
Chembiochem : a European journal of chemical biology, 14(2), 191-194 (2013-01-11)
Exploiting catalytic promiscuity: The proline-based enzyme 4-oxalocrotonate tautomerase (4-OT) promiscuously catalyzes asymmetric Michael-type additions of linear aldehydes--ranging from acetaldehyde to octanal--to trans-β-nitrostyrene in aqueous solvent. The presence of 1.4 mol% of 4-OT effected formation of the anticipated γ-nitroaldehydes in fair
Yan Huang et al.
The Journal of organic chemistry, 74(3), 1252-1258 (2008-12-31)
A convenient and efficient way for the highly diastereoselective synthesis of beta-substituted-alpha,gamma-diaminobutyric acids and pyrrolidines containing multichiral centers has been well-developed. Michael addition of chiral tricyclic iminolactones 1 and 2 to nitroalkenes afforded the adducts in good yields (up to
Hui Yang et al.
Organic & biomolecular chemistry, 10(16), 3229-3235 (2012-03-10)
(S)-Proline-catalyzed nitro-Michael additions of aldehydes and ketones to β-nitrostyrene were investigated computationally (MP2/6-311+G**//M06-2X/6-31G**). Contrary to what is usually assumed in organocatalysis, the lowest-energy transition states of proline-catalyzed nitro-Michael reactions do not necessarily involve the carboxylic acid group of the proline
Ellen Zandvoort et al.
Chembiochem : a European journal of chemical biology, 13(13), 1869-1873 (2012-08-02)
Serendipitous switch: While exploring cis-nitrostyrene as a potential electrophile in Michael-type addition reactions catalysed by the enzyme 4-oxalocrotonate tautomerase (4-OT), it was unexpectedly found that 4-OT catalyses the isomerisation of cis-nitrostyrene to trans-nitrostyrene (k(cat) /K(m) = 1.9×10(3) M(-1) s(-1) ).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service