M13807
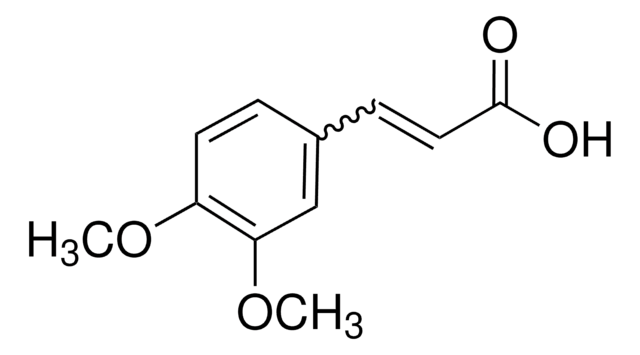
4-Methoxycinnamic acid, predominantly trans
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CH=CHCO2H
CAS Number:
Molecular Weight:
178.18
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
liquid crystal
mp
170-173 °C (lit.)
transition temp
crystalline phase to nematic phase 173.5 °C
nematic phase to isotropic phase 190 °C
SMILES string
COc1ccc(\C=C\C(O)=O)cc1
InChI
1S/C10H10O3/c1-13-9-5-2-8(3-6-9)4-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-4+
InChI key
AFDXODALSZRGIH-QPJJXVBHSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
N Ikemoto et al.
Carbohydrate research, 239, 11-33 (1993-02-01)
The microscale analytical method that is being developed in this group for the structure determination of oligosaccharides yields monosaccharide derivatives bearing two types of chromophores suitable for exciton-coupling, namely, 4-bromobenzoate (lambda max 245 nm) and 4-methoxycinnamate (lambda max 311 nm).
Sirichai Adisakwattana et al.
Life sciences, 78(4), 406-412 (2005-09-06)
We investigated the antihyperglycemic effect of p-methoxycinnamic acid (p-MCA), a cinnamic acid derivative, on plasma glucose and insulin concentrations, activities of hepatic glucose-regulating enzymes and hepatic glycogen content in normal and streptozotocin (STZ)-induced diabetic rats. p-MCA (10-100 mg/kg, PO) dose-dependently
H M Chawla et al.
Journal of photochemistry and photobiology. B, Biology, 105(1), 25-33 (2011-08-02)
A series of novel calix[4]arene enones (5-7) and cinnamates (12-14) have been synthesized and evaluated for ensuring protection from ultraviolet radiation (UVR). Spectroscopic analyses has revealed that compound 6 absorbs ultraviolet radiations between 280 and 350 nm with an absorption
Victor S Sobolev et al.
Journal of agricultural and food chemistry, 54(10), 3505-3511 (2006-05-17)
The peanut plant (Arachis hypogaea) is known to produce stilbene phytoalexins as a defensive response to fungal invasion; however, the distribution of phytoalexins among different organs of the peanut plant at early stages of growth under axenic conditions has not
Gunasekaran Sivagami et al.
Chemico-biological interactions, 196(1-2), 11-22 (2012-02-14)
We investigated the chemopreventive effect of p-methoxycinnamic acid (p-MCA), an active phenolic acid of rice bran, turmeric, and Kaemperfia galanga against 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Male albino Wistar rats were randomly divided into six groups. Group 1 consisted of control
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service