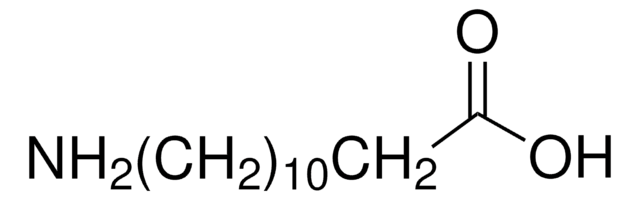A82605
11-Aminoundecanoic acid
97%
Synonym(s):
Aminoundecanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2(CH2)10CO2H
CAS Number:
Molecular Weight:
201.31
Beilstein:
1767291
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
white
mp
188-191 °C (lit.)
application(s)
peptide synthesis
SMILES string
NCCCCCCCCCCC(O)=O
InChI
1S/C11H23NO2/c12-10-8-6-4-2-1-3-5-7-9-11(13)14/h1-10,12H2,(H,13,14)
InChI key
GUOSQNAUYHMCRU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
11-Aminoundecanoic acid also known as aminoundecanoic acid, is utilized in solution phase peptide synthesis. It is also a monomer precursor for nylon-11.
Application
11-Aminoundecanoic acid can be used as a linker to synthesize amide-linked linear guanosine dimer.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Syntheses of 12-aminododecanoic and 11-aminoundecanoic acids from vernolic acid
Journal of the American Oil Chemists' Society, 74, 531-538 (1997)
Development of Minimal Diguanosinyl Motif toward RNA G-Quadruplex-Like Structures in Solution
Chembiochem, 21, 1837-1842 (2020)
M Marastoni et al.
European journal of medicinal chemistry, 35(6), 593-598 (2000-07-25)
The latent membrane protein 2 (LMP2) is expressed in EBV-associated tumours. LMP2 is a target of HLA-A2 restricted EBV-specific CTL responses and consequently it may represent a good target for specific CTL-based immunotherapies. However, the efficacy of such therapy is
Liling Zeng et al.
Nano letters, 5(10), 2001-2004 (2005-10-13)
Carboxylic acid-functionalized SWNTs prepared via the reaction of an amino acid, NH2(CH2)nCO2H, with fluoronanotubes show similar levels of sidewall functionalization; however, the solubility in water is controlled by the length of the hydrocarbon side chain (i.e., n). The 6-aminohexanoic acid
[Antibacterial effect of an ammonium salt of 11-aminoundecanoic acid. 5. Organic ammonium salts].
F Devínsky et al.
Die Pharmazie, 34(9), 574-574 (1979-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service